Information to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

December 2012 Number 1
Calochortiana December 2012 Number 1 December 2012 Number 1 CONTENTS Proceedings of the Fifth South- western Rare and Endangered Plant Conference Calochortiana, a new publication of the Utah Native Plant Society . 3 The Fifth Southwestern Rare and En- dangered Plant Conference, Salt Lake City, Utah, March 2009 . 3 Abstracts of presentations and posters not submitted for the proceedings . 4 Southwestern cienegas: Rare habitats for endangered wetland plants. Robert Sivinski . 17 A new look at ranking plant rarity for conservation purposes, with an em- phasis on the flora of the American Southwest. John R. Spence . 25 The contribution of Cedar Breaks Na- tional Monument to the conservation of vascular plant diversity in Utah. Walter Fertig and Douglas N. Rey- nolds . 35 Studying the seed bank dynamics of rare plants. Susan Meyer . 46 East meets west: Rare desert Alliums in Arizona. John L. Anderson . 56 Calochortus nuttallii (Sego lily), Spatial patterns of endemic plant spe- state flower of Utah. By Kaye cies of the Colorado Plateau. Crystal Thorne. Krause . 63 Continued on page 2 Copyright 2012 Utah Native Plant Society. All Rights Reserved. Utah Native Plant Society Utah Native Plant Society, PO Box 520041, Salt Lake Copyright 2012 Utah Native Plant Society. All Rights City, Utah, 84152-0041. www.unps.org Reserved. Calochortiana is a publication of the Utah Native Plant Society, a 501(c)(3) not-for-profit organi- Editor: Walter Fertig ([email protected]), zation dedicated to conserving and promoting steward- Editorial Committee: Walter Fertig, Mindy Wheeler, ship of our native plants. Leila Shultz, and Susan Meyer CONTENTS, continued Biogeography of rare plants of the Ash Meadows National Wildlife Refuge, Nevada. -

Federal Register/Vol. 63, No. 250/Wednesday, December 30
71838 Federal Register / Vol. 63, No. 250 / Wednesday, December 30, 1998 / Proposed Rules * * * * * streams or rivers in Cochise and Santa appointment, during normal business Dated: December 22, 1998. Cruz counties, Arizona. If this proposal hours at the above address. Donald Barry, is made final, section 7 of the Act would FOR FURTHER INFORMATION CONTACT: prohibit destruction or adverse Tom Assistant Secretary for Fish and Wildlife and Gatz, Endangered Species Coordinator, Parks. modification of critical habitat by any at the above address (telephone 602/ [FR Doc. 98±34412 Filed 12±23±98; 3:59 pm] activity funded, authorized, or carried 640±2720 ext. 240; facsimile 602/640± BILLING CODE 4310±55±C out by any Federal agency. Section 4 of the Act requires us to consider 2730). economic and other impacts of SUPPLEMENTARY INFORMATION: DEPARTMENT OF THE INTERIOR specifying any particular area as critical habitat. We solicit data and comments Background Fish and Wildlife Service from the public on all aspects of this Lilaeopsis schaffneriana ssp. recurva proposal, including data on the 50 CFR Part 17 (referred to as Lilaeopsis in this economic and other impacts of the proposed rule), the Huachuca water RIN 1018±AF37 designation. We may revise this umbel, is a plant found in cienegas proposal to incorporate or address new (desert marshes), streams and springs in Endangered and Threatened Wildlife information received during the southern Arizona and northern Sonora, and Plants; Proposed Determination of comment period. Mexico, typically in mid-elevation Critical Habitat for the Huachuca Water DATES: We will accept comments until wetland communities often surrounded Umbel, a Plant March 1, 1999. -

Arizona Game and Fish Department Heritage Data Management System
ARIZONA GAME AND FISH DEPARTMENT HERITAGE DATA MANAGEMENT SYSTEM Plant Abstract Element Code: PDAPI19051 Data Sensitivity: Yes CLASSIFICATION, NOMENCLATURE, DESCRIPTION, RANGE NAME: Lilaeopsis schaffneriana (Schlecht) var. recurva (A.W. Hill) Affolter COMMON NAME: Huachuca water umbel, Huachuca water-umbel, Huachuca waterumbel, Schaffner’s grasswort, Cienega False-rush SYNONYMS: Lilaeopsis recurva A.W. Hill, L. schaffneriana ssp. recurva FAMILY: Apiaceae AUTHOR, PLACE OF PUBLICATION: A.W. Hill, J. Linn. Soc. Bot. 47: 525-551. 1927. TYPE LOCALITY: Santa Cruz Valley near Tucson, Pima County, Arizona, U.S.A. TYPE SPECIMEN: LT: GH. C.G. Pringle s.n. 19 May 1881. LT: US. ST: NY, GH. TAXONOMIC UNIQUENESS: In the genus Lilaeopsis, the species schaffneriana is 1 of 5 species in North America, and contains only 1 variety recurva. According to Affolter (1985), “The genus Lilaeopsis Greene contains approximately 20 species. It is well developed in the temperate zones of North America, South America, Australia and New Zealand. 6 or 7 species recognized in North America.” According to NatureServe (2003), “The USFWS listed this taxon as Lilaeopsis schaffneriana ssp. recurva (Federal Register, Jan. 6, 1997). As of 11/31/99, L. schaffneriana var. recurva is used in its List of Endangered and Threatened Plants. The latter rank, is also used by Kartesz (1999). However, subspecies seems to be the rank used by Affolter (1985, p. 61), and is accepted in the Gray Index (online, 8/2000).” It is also used by the Missouri Botanical Garden (2003). DESCRIPTION: Herbaceous, semi-aquatic to aquatic perennial with cylindrical, wavy, yellowish green, slender hollow leaves borne individually or in clusters, that grow from the nodes of creeping rhizomes; inconspicuous septa at irregular intervals. -

ANNUAL T Tor Bertha V1nnond Deoember 1, 1936
Annual Report of Home Demonstration Agent, Cochise County 1937 Item Type text; Report Authors University of Arizona. Agricultural Extension Service. Home Demonstration Agents; Virmond, Bertha J. Publisher University of Arizona Rights Public Domain: This material has been identified as being free of known restrictions under U.S. copyright law, including all related and neighboring rights. Download date 02/10/2021 06:58:03 Item License http://creativecommons.org/publicdomain/mark/1.0/ Link to Item http://hdl.handle.net/10150/637478 ANNUAL .N A R RAT I V ERE p' 0 R T tor cocarss C OCNTY Bertha V1nnond C.cunty Home Demonstration Agent Deoember 1, 1936 to Deoember 1, 193'7 TABLE OF CONTENTS Page status of County Organization • · · • • • • • · 1 Program of Work. • · · · • • • • • 2 Homemakers Club s · • • • · • • • • • • • 3-4-5 Nutrition. • • • • · · · · · • · • 6 8c 23 Food Preparation • · • • • · • • .. 6 8c 23 Adult Work • · • · · • 6 4-H Baking Clubs • · · 23 4-H Meal Planning Club. • · . 26 Food Preservation • 7 8c 28 Adult . • · 7 4-H Canning Clubs · · · 28 . 9 Clothing • & 31 Adult Work. • • · · 9 4-H Garment Making Clubs. • · . 31 11 8c 45 Home Management • • 11 Adult Work. • • · • 45 4-H Home Management Club · 13 &. 48 Home FUrnishings . • · · · · • 13 Adult Work • • · · · · 48 4-H Handicraft Clubs • • . Health & . Home, Sanitation. 56 4-H Health Clubs · . 56 Miscellaneous • . 16 Adult Work . · . 16 w. P. A. Work . 16 Fairs • . · . · . 16 Organization · . 17 Club Houses . 17 Magazines • . · . · . 18 News Writing . 18 stewart Garden Club • · . 18 Willcox Woman's Club . 19 Cochise County History · . 19 Annual Leave • • . 20 4-H Club History •• · . 21 4-H Music Appreciation • · . -

United States Department of the Interior U.S. Fish and Wildlife
United States Department of the Interior U.S. Fish and Wildlife Service 2321 West Royal Palm Road, Suite 103 Phoenix, Arizona 85021 Telephone: (602) 242-0210 FAX: (602) 242-2513 AESO/SE 2-21-98-F-399-R1 October 24, 2002 Mr. John McGee, Forest Supervisor Coronado National Forest 300 West Congress, 6th Floor Tucson, Arizona 85701 RE: Reinitiation of Biological Opinion 2-21-98-F-399; Continuation of Livestock Grazing on the Coronado National Forest Dear Mr. McGee: This document transmits the U.S. Fish and Wildlife Service's (Service) final biological opinion and conference opinion on the proposed continuation of livestock grazing on the Coronado National Forest (Forest) in New Mexico (Hidalgo County) and Arizona (Cochise, Santa Cruz, Pima, Pinal, and Graham counties) pursuant to section 7 of the Endangered Species Act (Act) of 1973, as amended (16 U.S.C. 1531 et seq.). We appreciate the cooperation and assistance of your staff and permittees during the consultation period. We look forward to assisting you with the implementation of this biological opinion. If you have any questions on the biological opinion please contact Thetis Gamberg at 520/670-4619, Mima Falk at (520) 670-4550, or Sherry Barrett at (520) 670-4617 of my Tucson staff. Sincerely, /s/ Steven L. Spangle Field Supervisor 2 Enclosures: biological opinion zip disk cc: Assistant Regional Director, Fish and Wildlife Service, Albuquerque, NM (ARD-ES) (Attn: Sarah Rinkevich) Field Supervisor, Fish and Wildlife Service, Albuquerque, NM Refuge Manager, Fish and Wildlife Service, San Bernadino National Wildlife Refuge, Douglas, AZ Director, Arizona Game and Fish Department, Phoenix, AZ Regional Supervisor, Arizona Game and Fish Department, Tucson, AZ Director, New Mexico Department of Game and Fish, Albuquerque, NM CNF ReinG raze20 02Fina lBO.w pd:tatg Mr. -

R..Kix;:L' the GHIRICAHUA MOUNTAIN REGION
The mammals of the Chiricahua Mountain region, Cochise County, Arizona Item Type text; Thesis-Reproduction (electronic) Authors Maza, Bernardo George, 1931- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 26/09/2021 18:38:25 Link to Item http://hdl.handle.net/10150/551808 THE MAMMALS OF r..kix;:L' THE GHIRICAHUA MOUNTAIN REGION, COCHISE COUNTY, ARIZONA Bernardo G. Maza A Thesis Submitted to the Faculty of the DEPARTMENT OF ZOOLOGY In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 1965 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfillment of requirements for an advanced degree at The University of Arizona and is deposited in The University Library to be made available to borrowers under rules of the University Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in their judgment the proposed use of the material is in the interests of scholarship. In all other instances, however, permission must be ob tained from the author. APPROVAL BY THESIS DIRECTOR This thesis has been approved on the date shown below: E. -

Native Fishes and Natural Aquatic Habitats in Us Fish and Wildlife
•• NATIVE FISHES AND NATURAL AQUATIC HABITATS IN U. S. FISH AND WILDLIFE SERVICE REGION II WEST OF THE CONTINENTAL DIVIDE A Review of Population and Habitat Status and Evaluation of Survival Potentials for Native Freshwater Fishes, with Recommendations for Manaoement to Perpetuate the Indigenous Regional Fauna by W. L. MinckiPs,' Department of Zoolooy, Arizona State University, Tempe, Arizona 352S7 4 30 December 925 EXECUTIVE SUMMARY Natural aquatic habitats are becoming scarce in the arid American Southwest due to man's expanding needs for water. Native fishes are in direct competition for this resource, and are rapidly losing in their struggle for existance. Judicious husbandry of existing habitats, reclamation of those which remain relatively natural, and an intensive program of management of native fishes can stop and reverse current trends toward faunal extinction. Recommendations are to treat these fishes as faunal complexes, and define, delineate, and acquire for management those habitats with attributes suitable to support groups of species in perpetuity. Already-protected and geographically remote habitats are of top priority, those used by man but still in a natural or semi-natural state are second, and others requiring increasing levels of reclamation are decreasingly emphasized. Big rivers and their fishes are most critically endangered, streams and fish species of intermediate and high elevations are least so, and special habitats and fishes are manageable through site acquisition and reintroduction programs. There is not =.uffirient time for in-depth research prior to artinnq to recover many of the imperiled species and populations. Collection of basic information on reproduction, species interactions, and population dispersion and dispersal, must occur concurrently with habitat and species recovery and management operations. -
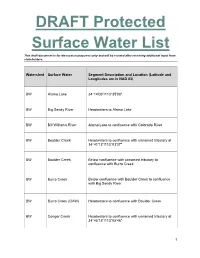
DRAFT Protected Surface Water List
DRAFT Protected Surface Water List This draft document is for discussion purposes only and will be revised after receiving additional input from stakeholders. Watershed Surface Water Segment Description and Location (Latitude and Longitudes are in NAD 83) BW Alamo Lake 34°14'06"/113°35'00" BW Big Sandy River Headwaters to Alamo Lake BW Bill Williams River Alamo Lake to confluence with Colorado River BW Boulder Creek Headwaters to confluence with unnamed tributary at 34°41'13"/113°03'37" BW Boulder Creek Below confluence with unnamed tributary to confluence with Burro Creek BW Burro Creek Below confluence with Boulder Creek to confluence with Big Sandy River BW Burro Creek (OAW) Headwaters to confluence with Boulder Creek BW Conger Creek Headwaters to confluence with unnamed tributary at 34°45'15"/113°05'46" 1 This draft document is for discussion purposes only and will be revised after receiving additional input from stakeholders. BW Conger Creek Below confluence with unnamed tributary to confluence with Burro Creek BW Copper Basin Wash Headwaters to confluence with unnamed tributary at 34°28'12"/112°35'33" BW Cottonwood Canyon Headwaters to Bear Trap Spring BW Cottonwood Canyon Below Bear Trap Spring to confluence at Smith Canyon Sycamore Creek BW Date Creek Headwaters to confluence with Santa Maria River BW Francis Creek (OAW) Headwaters to confluence with Burro Creek BW Kirkland Creek Headwaters to confluence with Santa Maria River BW Knight Creek Headwaters to confluence with Big Sandy River BW Peeples Canyon Headwaters to confluence with Santa Maria River (OAW) BW Santa Maria River Headwaters to Alamo Lake BW Trout Creek Headwaters to confluence with unnamed tributary at 35°06'47''/113°13'01'' 2 This draft document is for discussion purposes only and will be revised after receiving additional input from stakeholders. -

Turner and List: Arizona Native Fish Streams 1 Habitat Mapping And
Turner and List: Arizona native fish streams This is a preprint of an article published in Aquatic Conservation: Marine and Freshwater Ecosystems, Vol. 17: 737-748 (2007). http://www.interscience.wiley.com Habitat mapping and conservation analysis to identify critical streams for Arizona’s native fish Dale S. Turner* and Michael D. List The Nature Conservancy 1510 E. Fort Lowell Road Tucson, AZ 85719, USA *Correspondence: Dale S. Turner The Nature Conservancy 1510 E. Fort Lowell Road Tucson, AZ 85719, USA E-mail: [email protected] Telephone: 520-622-3861 Fax: 520-620-1799 Running title: Arizona native fish streams 1 Turner and List: Arizona native fish streams ABSTRACT 1. Arizona’s native fish species are among the most imperiled fauna in North America. Knowing the current distribution of native fish and their habitat is critical to their management and conservation, but the last detailed mapping effort was more than 30 years ago and predated computer mapping techniques. 2. Current distribution of 34 native fish species was modeled by identifying perennial stream segments for which species presence had been documented. A composite of these single-species maps displays a pattern of species richness that can inform conservation, especially when overlaid with maps of management status or invasive species. 3. The map overlays suggest that conservation priorities should include Eagle Creek, the Verde River and its tributaries, Aravaipa Canyon, the Virgin River, and Black Draw, which together hold 63% of native fish species. Of the 32 streams which support 5 or more native species, 28 have at least one non-native fish species, indicating that a more aggressive program of removing non-natives may be critical to maintaining those native populations. -

Section VII Potential Linkage Zones SECTION VII POTENTIAL LINKAGE ZONES
2006 ARIZONA’S WILDLIFE LINKAGES ASSESSMENT 41 Section VII Potential Linkage Zones SECTION VII POTENTIAL LINKAGE ZONES Linkage 1 Linkage 2 Beaver Dam Slope – Virgin Slope Beaver Dam – Virgin Mountains Mohave Desert Ecoregion Mohave Desert Ecoregion County: Mohave (Linkage 1: Identified Species continued) County: Mohave Kit Fox Vulpes macrotis ADOT Engineering District: Flagstaff and Kingman Mohave Desert Tortoise Gopherus agassizii ADOT Engineering District: Flagstaff ADOT Maintenance: Fredonia and Kingman Mountain Lion Felis concolor ADOT Maintenance: Fredonia ADOT Natural Resources Management Section: Flagstaff Mule Deer Odocoileus hemionus ADOT Natural Resources Management Section: Flagstaff Speckled Dace Rhinichthys osculus Spotted Bat Euderma maculatum AGFD: Region II AGFD: Region II Virgin Chub Gila seminuda Virgin Spinedace Lepidomeda mollispinis mollispinis BLM: Arizona Strip District Woundfin Plagopterus argentissimus BLM: Arizona Strip District Congressional District: 2 Threats: Congressional District: 2 Highway (I 15) Council of Government: Western Arizona Council of Governments Urbanization Council of Government: Western Arizona Council of Governments FHWA Engineering: A2 and A4 Hydrology: FHWA Engineering: A2 Big Bend Wash Legislative District: 3 Coon Creek Legislative District: 3 Virgin River Biotic Communities (Vegetation Types): Biotic Communities (Vegetation Types): Mohave Desertscrub 100% Mohave Desertscrub 100% Land Ownership: Land Ownership: Bureau of Land Management 59% Bureau of Land Management 93% Private 27% Private -

Toward Integrated Research, Land a 13.151/5:RMRS-P-10 .Z-=A Department Of
This file was created by scanning the printed publication. Errors identified by the software have been corrected; however, some errors may remain. USDA U nited States Toward Integrated Research, Land A 13.151/5:RMRS-P-10 .z-=a Department of USDA United States Agriculture Agriculture Forest Service Management, and Ecosystem Forest Service Rocky Mountain Rocky Mountain Research Station Research Station Proceedings Protection in the Malpai Borderlands: RMRS-P-10 Proceedings |une1999 RMRS-P-10 Toward Integrated Research, Land june 1999 Conference Summary Management, and Ecosystem Protection in the Malpai Borderlands: Conference Summary to*/"*) January 6-8, 1999 KM U. -1- i) -• , . - ' Douglas, Arizona .„- , t ""'-'•--'.«-t* ' '' ' •, * --•'*. ,.' --*,. * t.- : w. - Illu^- January 6-8, 1999 Douglas, Arizona -:>>4«^ -k :^;; -.^>^ '.m Gottfried, Gerald J.; Eskew, Lane G.; Curtin, Charles G.; and Edminster, Carleton B., compilers. 1999. Toward integrated research, land management, and ecosystem protection in the Malpai Borderlands: Conference summary; 6-8 January 1999; Douglas, AZ. Proceedings RMRS-P-10. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 136 p. Abstract Land management based on sound science is key to increased productivity and bio logical diversity along the U.S./Mexico border in southeastern Arizona and south western New Mexico. The USDA Forest Service's Rocky Mountain Research Station and the non-government Malpai Borderlands Group have sponsored studies andre source inventories in the region. A meeting in Douglas, Arizona, was held to inform the scientific, land management, and local communities of progress. These proceed ings contain abstracts from the presentations. -

Annual Narrative Report
Annual Report of Home Demonstration Agent, Cochise County 1938 Item Type text; Report Authors University of Arizona. Agricultural Extension Service. Home Demonstration Agents; Virmond, Bertha J. Publisher University of Arizona Rights Public Domain: This material has been identified as being free of known restrictions under U.S. copyright law, including all related and neighboring rights. Download date 04/10/2021 03:03:54 Item License http://creativecommons.org/publicdomain/mark/1.0/ Link to Item http://hdl.handle.net/10150/637479 ANNUAL NARRATIVE REPORT tor .coomBE COUNTY Bertha Virmond County Home Demonstration Agent Decanber 1, 1937 to December 1, 1938 TABU: OF CONTENTS Page • • • status of County Organization • • • • • • • • • • • • 1 • • Program 0'1 Work • • • • • • • • • • • • • • • • • • • • 2 Homemakers Clubs ••••••••••••••••••••• 3-4 Nutrition • • • • • • • • • • • • • • • • • • • • • • • • • 5 &. 23 Food Preparation • • • • • • • • • • • • • • • • • • • 5 &. 23 .. Adult Work • • • • • • • • • • • • • • • • • • • • • 5 6 4� Baking Clubs • • • • • • • • • • • • • • • • • • 23 - 25 Food Preservation • • • • • • • • • • • • • • • • • • • 'I &. 26 - Adult ••• • • • •••••••••••••••••• 'I 8 4� Canning Clubs •••••••••••••••••• 26 - 2'1 • • • • • • • • 9 &. 28 Clothing • • • • • • • • • • • • • • • • • • - Adult Work •••• . • • • • • ••••••••••• 9 10 28 - 40 4� Garment Making Clubs • • • • • • • • • • • • •••• • • • • • •• 11 Home KaDagement • • • • • • • • • • • • • • • • 11 12 Adult Work • • • • • • • • • • • • • •••••••••• 43 • • •