Accepted Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vegetation and Flora of Auyuittuq National Park Reserve, Baffin Island
THE VEGETATION AND FLORA OF AUYUITTOQ NATIONAL PARK RESERVE, BAFFIN ISLAND ;JAMES E. HINES AND STEVE MOORE DEPARTMENT OF RENEWABLE RESOURCES GOVERNMENT OF THE NORTHWEST TERRITORIES YELLOWKNIFE I NORTHWEST TERRITORIES I XlA 2L9 1988 A project completed under contract to Environment Canada, Canadian Parks Service, Prairie and Northern Region, Winnipeg, Manitoba. 0 ~tona Renewable Resources File Report No. 74 Renewabl• R•sources ~ Government of tha N p .0. Box l 310 Ya\lowknif e, NT XlA 2L9 ii! ABSTRACT The purposes of this investigation were to describe the flora and major types of plant communities present in Auyuittuq National Park Reserve, Baffin Island, and to evaluate factors influencing the distribution of the local vegetation. Six major types of plant communities were recognized based on detailed descriptions of the physical environment, flora, and ground cover of shrubs, herbs, bryophytes, ·and lichens at 100 sites. Three highly interrelated variables (elevation, soil moisture, and texture of surficial deposits) seemed to be important in determining the distribution and abundance of plant communities. Continuous vegetation developed mainly at low elevations on mesic to wet, fine-textured deposits. Wet tundra, characterized by abundant cover of shrubs, grasses, sedges, and forbs, occurred most frequently on wet, fine-textured marine and fluvial sediments. Dwarf shrub-qram.inoid comm.unities were comprised of abundant shrubs, grasses, sedges and forbs and were found most frequently below elevations of 400 m on mesic till or colluvial deposits. Dwarf shrub comm.unities were characterized by abundant dwarf shrub and lichen cover. They developed at similar elevations and on similar types of surficial deposits as dwarf-shrub graminoid communities. -

Lichens and Vascular Plants in Duvefjorden Area on Nordaust- Landet, Svalbard
CZECH POLAR REPORTS 9 (2): 182-199, 2019 Lichens and vascular plants in Duvefjorden area on Nordaust- landet, Svalbard Liudmila Konoreva1*, Mikhail Kozhin1,2, Sergey Chesnokov3, Soon Gyu Hong4 1Avrorin Polar-Alpine Botanical Garden-Institute of Kola Scientific Centre of RAS, 184250 Kirovsk, Murmansk Region, Russia 2Department of Geobotany, Faculty of Biology, Lomonosov Moscow State University, Leninskye Gory 1–12, GSP–1, 119234 Moscow, Russia 3Komarov Botanical Institute RAS, Professor Popov St. 2, 197376 St. Petersburg, Russia 4Division of Polar Life Sciences, Korea Polar Research Institute, 26, Songdomirae-ro, Yeonsu-gu, Incheon 21900, Republic of Korea Abstract Floristic check-lists were compiled for the first time for Duvefjorden Bay on Nordaust- landet, Svalbard, based on field work in July 2012 and on data from literature and herbaria. The check-lists include 172 species of lichens and 51 species of vascular plants. Several species rare in Svalbard and in the Arctic were discovered: Candelariella borealis was new to Svalbard. 51 lichen species were newly recorded on Nordaustlandet and 131 lichen species were observed in the Duvefjorden area for the first time. Among lichen species rare in Svalbard and in the Arctic the following can be mentioned: Caloplaca magni-filii, C. nivalis, Lecidea silacea, Phaeophyscia nigricans, Polyblastia gothica, Protothelenella sphinctrinoidella, Rinodina conradii, Stenia geophana, and Tetramelas pulverulentus. Two species of vascular plants, Saxifraga svalbardensis and S. hyperborea, were found new to the Duvefjorden area. The investigated flora is represented mostly by species widespread in Svalbard and in the Arctic. Although Duvefjorden area is situated in the northernmost part of Svalbard, its flora is characterized by relatively high diversity of vascular plants and lichens. -

General Ecology and Vascular Plants of the Hazencamp Area* D
GENERAL ECOLOGY AND VASCULAR PLANTS OF THE HAZENCAMP AREA* D. B. 0. Savile General description AZEN CAMP,at 81”49’N., 71”18W., lies on a small sandy point on the H northwest shore of Lake Hazen, in northeast Ellesmere Island. Lake Hazen stands at 158 m. above sea-level, extends 78 km. ENE to WSW, and has a maximum width of 11 km. It lies at the northern edge of a plateau bounded on the south by the Victoria and Albert Mountains and on the north by the United States Range. The Garfield Range, a southern outlier of the United States Range, extends to within 4 km. of Hazen Camp. The high mountain ranges and icefields, with extensive areas over 2000 m., and smaller hills effectively protect the land about Lake Hazen from incursions of unmodified cold air, and induce a summer climate that is very exceptional for this latitude. On the other hand the lake itself is large enough to keep air temperatures adjacent to the shore appreciably below those prevailing a few kilometres away. The geologyof northeasternEllesmere Island has recently been described by Christie (1962). The lowlands at Hazen Camp are underlain by Mesozoic and Permian sediments, mainly sandstone and shale. These sediments outcrop conspicuously on Blister Hill (altitude 400 m.), 1.5 to 2.7 km. west of the camp; andon a seriesof small but steep foothills running southwest to northeast along a fault and passing 2.5 to 3 kn. northwest of the camp. These rocks weather rapidly. Consequently sand and clay in varying proportions are plentiful in the camp area. -

Skrifter Om·Svalbard Og Ishavet
DET KONGELIGE DEPARTEMENT FOR HANDEL , SJØFART, INDUSTRI, HANDVERK OG FISKERI NORGES SVALBARD- OG ISHAVS-UNDERSØKELSER LEDER: ADOLF HOEL SKRIFTER OM·SVALBARD OG ISHAVET Nr. 62 P. F. SCHOLANDER VASCULAR PLANTS FROM NORTHERN SVALBARD WITH REMARKS ON THE VEGETATION IN NORTH-EAST LAND WITH 61 FIGUR ES IN THE TEXT, 5 PLATES AND 2 lIIAPS (SCIENTIFIC RESULTS OF THE SWEDISH-NORWEGIAN ARCTIC EXPEDITION IN THE SUMMER OF 1931. PART XIII) ---(t--- OSLO I KOMMISJON HOS JACOB DYBWAD 1934 DET KONGELIGE DEPARTEMENT FOR HANDEL, SJ0FART, INDUSTRI, HANDVERK OG FISKERI NORGE S SVALBARD- OG ISHAV S-UNDER S0KE LSER LEDER: ADOLF HOEL SKRIFTER OM SVALBARD OG ISHAVET Nr. 62 P. F. SCHOLANDER VASCULAR PLANTS FROM NORTHERN SVALBARD WITH REMAR KS ON TH E VEG ETATION IN NORTH- EAST LAND WITH 61 FIGURES IN THE TEXT, 5 PLATES AND 2111APS (SCIENTIFIC RESULTS OF THE SWEDISH-NORWEGIAN ARCTIC EXPEDITION IN THE SUMMER OF 1931. PART XIII) OSLO I KOMMISJON HOS JACOB DYBWAD 1934 PRI NTED WITH CONTRIBUTION FROM ANDREEFONDET AND NANSEN FONDET A. W. BR0GGERS BOKTRYKKERI A!S Contents. Page Preface. .. .. .. .. .. .. ..... , .. .. .. .. 5 I. Botanical Investigations in Northern Svalbard....... 7 If. List of Botanical Localities............................................. 11 IlL Enumeration of Vascular Plants and their Distribution. ...... .. .... .. '" 15 Pteridophyta. .. .. .. .. ... 15 Dicotyledones .. .. .. .. ...... .... ...... .. .. ... ......... ... ... " 16 Monocotyledones. .... .. ..... .... .. .... .. .. ... .. ... ... ... ... ...... 54 Short survey of some of the more important finds, with a list of the species reaching their northern limit in the area investigated 104 List of some vascular plants collected in West Spitsbergen and Frans J osef Land. .. .. .. .. ... .................. 105 IV. Remarks on the Vegetation in North-East Land . ........ .. .... ....... 107 A. Vegetation of the non-dolomitic areas. -

Arctic Vegetation Archive (AVA) Workshop Krakow, Poland, April 14-16, 2013 Credits
p u o r G C a A or FF Fl CAFF Proceeding Series Report Nr. 10 September 2013 Arctic Vegetation Archive (AVA) Workshop Krakow, Poland, April 14-16, 2013 Credits CAFF Designated Agencies: • Directorate for Nature Management, Trondheim, Norway • Environment Canada, Ottawa, Canada • Faroese Museum of Natural History, Tórshavn, Faroe Islands (Kingdom of Denmark) • Finnish Ministry of the Environment, Helsinki, Finland • Icelandic Institute of Natural History, Reykjavik, Iceland • The Ministry of Housing, Nature and Environment, Government of Greenland • Russian Federation Ministry of Natural Resources, Moscow, Russia • Swedish Environmental Protection Agency, Stockholm, Sweden • United States Department of the Interior, Fish and Wildlife Service, Anchorage, Alaska CAFF Permanent Participant Organizations: • Aleut International Association (AIA) • Arctic Athabaskan Council (AAC) • Gwich’in Council International (GCI) • Inuit Circumpolar Council (ICC) – Greenland, Alaska and Canada • Russian Indigenous Peoples of the North (RAIPON) • Saami Council This publication should be cited as: Walker, D.A. Breen, A.L., Raynolds, M.K. & Walker, M.D. (Ed). 2013. Arctic Vegetation Archive (AVA) Workshop, Krakow, Poland, April 14-16, 2013. CAFF Proceedings Report #10. Akureyri, Iceland. ISBN: 978-9935-431-24-0 Cover photo: Fred Daniëls sampling a wet relevé plot in the high Arctic at Isachsen, Ellef Ringnes Island, Canada. Dominant species in the relevé include Luzula nivalis, Alopecurus alpinus, Schistidium holmenianum, Oncophorus wahlenbergii, Aulacomnium turgidum, Polytrichastrum alpinum, Collema ceraniscum, and Lecidea ramulosa Photo: D.A. Walker, July 2005. Back cover photo: Snowbed vegetation in the high Arctic at Isachsen, Ellef Ringnes Island, Canada. Photo: Fred Daniëls, July 2005. For more information please contact: CAFF International Secretariat Borgir, Nordurslod 600 Akureyri, Iceland Phone: +354 462-3350 Fax: +354 462-3390 Email: [email protected] Internet: www.caff.is Editing: D.A. -

Volume 2, Chapter 18-1: Large Mammals: Ruminants-Cervidae
Glime, J. M. 2018. Large Mammals: Ruminants – Cervidae. Chapter 18-1. In: Glime, J. M. Bryophyte Ecology. Volume 2. 18-1-1 Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 18-1 LARGE MAMMALS: RUMINANTS - CERVIDAE TABLE OF CONTENTS Ruminantia – Ruminants ...................................................................................................................................................... 18-1-2 Impact of Ruminants on Bryophytes .................................................................................................................................... 18-1-4 Grazing ........................................................................................................................................................................ 18-1-4 Trampling ................................................................................................................................................................... 18-1-12 Manuring .................................................................................................................................................................... 18-1-14 Life on Manure – Splachnaceae ......................................................................................................................................... 18-1-15 Cervidae – Deer, Elk, Moose, and Caribou ....................................................................................................................... -
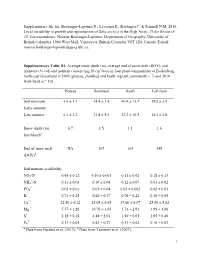
Supplementary File For: Boulanger-Lapointe N., Lévesque E., Baittinger C
Supplementary file for: Boulanger-Lapointe N., Lévesque E., Baittinger C. & Schmidt N.M. 2016. Local variability in growth and reproduction of Salix arctica in the High Arctic. Polar Research 35. Correspondence: Noémie Boulanger-Lapointe: Department of Geography, University of British Columbia, 1984 West Mall, Vancouver, British Columbia V6T 1Z4, Canada. E-mail: [email protected] Supplementary Table S1. Average snow depth (m), average end of snow melt (DOY), soil moisture (% vol) and nutrient content (µg/10 cm2/day) in four plant communities at Zackenberg, north-east Greenland in 2005 (plateau, snowbed and heath; n/plant community = 7) and 2010 (Fell-field, n = 10). Plateau Snowbed Heath Fell-field Soil moisture 4.0 ± 1.7 34.4 ± 7.8 40.9 ± 11.7 19.8 ± 3.5 Early summer Late summer 6.1 ± 2.2 21.8 ± 5.6 32.2 ± 10.5 14.3 ± 2.8 Snow depth (m) – 0.7 1.5 1.1 1.6 late Marcha End of snow melt NA 167 163 148 (DOY)b Soil nutrient availability NO3-N 0.48 ± 0.12 0.10 ± 0.001 0.11 ± 0.02 0.18 ± 0.13 + NH4 -N 0.11 ± 0.03 0.10 ± 0.04 0.12 ± 0.07 0.01 ± 0.02 3- PO4 0.02 ± 0.01 0.03 ± 0.04 0.01 ± 0.002 0.02 ± 0.01 K+ 0.71 ± 0.25 0.66 ± 0.37 0.76 ± 0.22 0.30 ± 0.05 Ca2+ 32.56 ± 6.12 25.65 ± 6.65 17.60 ± 8.07 25.50 ± 5.02 Mg2+ 7.77 ± 1.20 10.70 ± 3.05 5.74 ± 2.81 3.98 ± 1.00 S2- 2.18 ± 0.18 4.48 ± 2.01 1.90 ± 0.05 1.05 ± 0.40 Fe3+ 0.13 ± 0.02 0.43 ± 0.27 0.11 ± 0.02 0.10 ± 0.02 a b Data from Gacitua et al. -

List of Alaskan Seed Plant and Fern Names Compiled from ALA, ACCS, PAF, WCSP, FNA
List of Alaskan Seed Plant and Fern names Compiled from ALA, ACCS, PAF, WCSP, FNA (Questions to [email protected]) January 18, 2020 Acoraceae Acorus americanus (Raf.) Raf. (GUID: trop-2100001). In Alaska according to ALA, FNA. An accepted name according to ALA, FNA.A synonym of: • Acorus calamus var. americanus Raf. according to WCSP ; Comments: WCSP Acorus calamus L. (GUID: ipni-84009-1). In Alaska according to ACCS. An accepted name according to WCSP, ACCS. Adoxaceae Adoxa moschatellina L. (GUID: ipni-5331-2). In Alaska according to ALA, PAF, ACCS. An accepted name according to ALA, PAF, WCSP, ACCS. Sambucus pubens Michx. (GUID: ipni-227191-2). In Alaska according to ACCS. An accepted name according to WCSP, ALA.A synonym of: • Sambucus racemosa subsp. pubens (Michx.) Hultén according to PAF ; Comments: Subspecies [pubens] reaches the Arctic in southwestern Alaska. This hardy race is introduced in northern Norway and Iceland, escaping in Iceland, and may be found in the arctic parts. • Sambucus racemosa subsp. pubens (Michx.) House according to ACCS ; Comments: Panarctic Flora Checklist Sambucus racemosa L. (GUID: ipni-30056767-2). In Alaska according to ACCS. An accepted name according to ALA, WCSP, ACCS. Sambucus racemosa subsp. pubens (Michx.) House (GUID: NULL). In Alaska according to ACCS. An accepted name according to ACCS. Sambucus racemosa subsp. pubens (Michx.) Hultén (GUID: ipni-227221-2). In Alaska according to ALA, PAF. An accepted name according to ALA, PAF. Viburnum edule (Michx.) Raf. (GUID: ipni-149665-1). In Alaska according to ALA, PAF, ACCS. An accepted name according to ALA, PAF, ACCS. Viburnum opulus L. -

Nordic Journal of Botany NJB-01721
Nordic Journal of Botany NJB-01721 Philipp, M., Hansen, K., Monrad, D., Adsersen, H., Bruun, H. H. K. and Nordal, I. 2018. Hidden biodiversity in the Arctic – a study of soil seed banks at Disko Island, Qeqertarsuaq, West Greenland. – Nordic Journal of Botany doi: 10.1111/njb.01721 Appendix 1 Table A1. Plant species occurring in soil seed bank samples and in the vegetation above. The species are ordered after their presence in the soil seed bank and alphabetically. Species name Species without Non-clonal = 0 Seed without Woody = 0 Seed seed bank = 0 Clonal = 1 appendages = 0 Herbaceous = 1 weight Species with Seed with (mg) seed bank = 1 appendages = 1 Arabis alpina 1 0 0 1 0.24 a Arnica angustifolia 1 1 1 1 0.72 a Cardamine bellidiflora 1 0 0 1 0.17 b Cerastium alpinum 1 1 0 1 0.24 c Draba arctogena 1 0 0 1 Draba nivalis 1 0 0 1 0.03 a Draba subcapitata 1 0 0 1 0.23 c Festuca bracyphylla 1 0 0 1 0.71 c Luzula confusa 1 1 0 1 0.22 a Minuartia rubella 1 0 0 1 0.06 c Papaver radicatum 1 0 0 1 0.12 c Poa alpina 1 1 0 1 0.40 d Poa glauca 1 0 0 1 0.15 a Polygonum viviparum (bulbil) 1 1 0 1 0.85 c Potentilla vahliana 1 0 0 1 0.52 a Rumex acetosella 1 1 0 1 0.73 d Sagina saginoides 1 0 0 1 0.02 d Saxifraga cespitosa 1 0 0 1 0.05 a Saxifraga cernua (bulbil) 1 1 0 1 0.34 c Saxifraga nivalis 1 0 0 1 0.04 a Saxifraga oppositifolia 1 0 0 1 0.09 d Saxifraga rivularis 1 1 0 1 0.03 a Saxifraga tricuspidata 1 0 0 1 0.05 a Sibbaldia procumbens 1 1 0 1 0.45 a Stellaria longipes 1 1 0 1 0.17 a Taraxacum sp. -

The Genus Pleospora S. 1. from Svalbard
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Sydowia Jahr/Year: 1993 Band/Volume: 45 Autor(en)/Author(s): Holm Lennart, Holm K. Artikel/Article: The genus Pleospora s. l. from Svalbard. 167-187 ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at The genus Pleospora s. 1. from Svalbard L. Holm & K. Holm Dept. of Systematic Botany, University of Uppsala, Villavägen 6, S-75236 Uppsala, Sweden Holm, L. & K. Holm (1993). The genus Pleospora s. 1. from Svalbard. - Sydowia 45(2): 167-187. A critical account is given of the thirteen species of Pleospora s. 1. recognized from Svalbard, viz. Cilioplea coronata, Montagnula spinosella, Pleospora androsa- ces, P. arctica, P. ascodedicata, P. comata, P. glacialis, P. helvetica, P. penicillus, P. spetsbergensis, P. wulffii, Pyrenophora raetica, and Pyr. subalpina. Pleospora arc- tica Karst, is reinstated for P. arctagrostidis Oud. It is not a synonym of P. islandica Johans., as generally believed. The latter species is not known from Svalbard, but a very close ally is P. wulffii Lind. Several species recorded from Svalbard by Lind have not been found in this study, and the records seem doubtful. An obvious trait of Svalbard Pleosporae is the tendency towards the reduction of the setae. These are considered to have a protective function against mycophagous arthropoda, and their reduction in Svalbard collections may be related to the paucity of these animals the- re. Keywords: Taxonomy, floristic study, Pleosporales, Ascomycetes. During the ISAM III Symposium on Svalbard, 11-20 August 1988, several ascomycetes were collected. -

The History of the Flora of Spitsbergen and Bear Island and the Age of Some Arctic Plant Species
PRE SL I A 1960 32 : 225-253 Emil Had a c: The History of the Flora of Spitsbergen and Bear Island and the Age of some Arctic Plant Species Preface Since DARWIN's eminent work, the problem of the Origin of Species has not lost its attraction and interest. In the last years the discussion on this problem has revived very vigorously. As we are not able to give a definition of the Species acceptable for the majority of scientists, as well as an universal method for distinguishing a species from a variety or a genus, it is difficult to find a solid platform for the solution of this question. In this situation we must try to find at least some material to elucidate some ways which led to the origin of one or another wellknown species and hope, that our little contribution will throw some light on the ge neral problem. There are several methods, which can help us: the physiology, the ecology, the cytotaxonomy, etc. One of them, used already by Darwin him self, is the plant geography. During the last twenty years, the present author has studied the flora and vegetation of the European Arctic and Subarctic regions, and the mountain flora of Central Europe. Some of his studies have been already published; in this treatise he will try to summarize some results of his work. The first explorers of the Arctic thought that its flora came to this t erritory from the south in the postglacial. Another explanation of its occurrence was in this time unthinkable. -

North American Terrestrial Vegetation Second Edition
North American Terrestrial Vegetation Second Edition Edited by Michael G. Barbour William Dwight Billings PUBLISHED BY THE PRESS SYNDICATE OF THE UNIVERSITY OF CAMBRIDGE The Pitt Building, Trumpington Street, Cambridge, United Kingdom CAMBRIDGE UNIVERSITY PRESS The Edinburgh Building, Cambridge CB2 2RU, United Kingdom www.cup.cam.ac.uk 40 West 20th Street, New York, NY 10011-4211, USA www.cup.org 10 Stamford Road, Oakleigh, Melbourne 3166, Australia Ruiz de AlarcoÂn 13, 28014 Madrid, Spain q Cambridge University Press 1988, 1999 This book is in copyright. Subject to statutory exception and to the provisions of relevant collective licensing agreements, no reproduction of any part may take place without the written permission of Cambridge University Press. First published 1988 Second edition 1999 Printed in the United States of America Typeface, 9/11 Palatino pt. System DeskTopPro/UX [RF] A catalog record for this book is available from the British Library. Library of Congress Cataloging-in-Publication Data North American terrestrial vegetation / edited by Michael G. Barbour, William Dwight Billings. ± 2nd ed. p. cm. Includes bibliographical references (p. ) and index. ISBN 0-521-55027-0 (hardbound) 1. Plant communities ± North America. 2. Plant ecology ± North America. 3. Phytogeography ± North America. I. Barbour, Michael G. II. Billings, W. D. (William Dwight), 1910±1997. QK110.N854 1999 581.7©22©097 ± dc21 97-29061 CIP ISBN 0 521 55027 0 hardback ISBN 0 521 55986 3 paperback Contents Contributors page vii Preface to the First Edition ix Preface to the Second Edition xi CHAPTER 1 Arctic Tundra and Polar Desert Biome 1 Lawrence C.