Chemical Industry Sangrur Hazardous Waste NGT.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dr. Shahila Zafar
Dr. Shahila Zafar Assistant Professor Department of Languages and Comparative Literature , School of Language, Literature and Culture Central University of Punjab, Bathinda, India , 151001 [email protected] ; [email protected] ; +91 - 9952112920 RESEARCH AND TEACHING INT E RESTS Applied Linguistics, Second Language Acquisition, Language Learning and Technology , English Language Teaching , African - American Literature , Indian Writing in English EDUCA T ION 2012 Ph.D. , Applied Linguistics , VIT University, Vellore , Tamil Nadu, India Advisor: Dr. K. Meenakshi Thesis Topic: Relationship between English Language Proficiency and Extrav ersion – Introversion Tendencies among University Level Chinese Students 2008 M.Phil. English, Madurai Kamraj University, Madurai , Tamil Nadu, India 2001 M . A . English , Punjabi University, Patiala , Punjab, India 1999 B . A . English ( Hons.) , Government College for Girls, Punjabi University Patiala , Punjab, India NON - DEGREE COURSES Courses completed at the Five Colleges, University of Massachusetts, Amherst, USA , 2016 - 17 1. Language Method - Colloquium 4. Broadcast News Reporting 2. Language Method - Practicum 5. Black Women Writers 3. Translation and Technology QUALIFYING EXAMS CLEARED 2001 - UGC (NET) - University Grants Commission’s National Eligibility Test for Lectureship in Engl ish Conducted by UGC, New Delhi, India. 2001 - State Level Eligibility Test (SLET) for Lectureship in English, conducted by Rajasthan Public Service Commission, Rajasthan, India TEACHING Assistant -

(OH) Category 1 14 Muhammad Sahib Town- Malerkotla, Distt
Department of Local Government Punjab (Punjab Municipal Bhawan, Plot No.-3, Sector-35 A, Chandigarh) Detail of application for the posts of Beldar, Mali, Mali-cum-Chowkidar, Mali -cum-Beldar- cum-Chowkidar and Road Gang Beldar reserved for Disabled Persons in the cadre of Municipal Corporations and Municipal Councils-Nagar Panchayats in Punjab Sr. App Name of Candidate Address Date of Birth VH, HH, No. No. and Father’s Name OH etc. Sarv Shri/ Smt./ Miss %age of disability 1 2 3 4 5 6 Orthopedically Handicapped (OH) Category 1 14 Muhammad Sahib Town- Malerkotla, Distt. 01.10.1998 OH 50% S/o Muhammad Shafi Sangrur 2 54 Harjinder Singh S/o Vill. Kalia, W.No.1, 10.11.1993 OH 55% Gurmail Singh Chotian, Teh. Lehra, Distt. Sangrur, Punjab. 3 61 Aamir S/o Hameed W.No.2, Muhalla Julahian 08.11.1993 OH 90% Wala, Jamalpura, Malerkotla, Sangrur 4 63 Hansa Singh S/o Vill. Makror Sahib, P.O. 15.10.1982 OH 60% Sham Singh Rampura Gujjran, Teh. Moonak, Distt. Sangrur, Punjab. 5 65 Gurjant Singh S/o Vill. Kal Banjara, PO Bhutal 02.09.1985 OH 50% Teja Singh Kalan, Teh. Lehra, Distt. Sangrur 6 66 Pardeep Singh S/o VPO Tibba, Teh. Dhuri, 15.04.1986 OH 60% Sukhdev Singh Distt. Sangrur 7 79 Gurmeet Singh S/o # 185, W. No. 03, Sunam, 09.07.1980 OH 60% Roshan Singh Sangrur, Punjab. 8 101 Kamaljit Singh S/o H. No.13-B, Janta Nagar, 09.08.1982 OH 90% Sh. Charan Singh Teh. Dhuri, Distt. -

Police Station.Pdf
LIST OF POLICE STATIONS ALLOTED TO THE ADDITIONAL DISTRICT & SESSIONS JUDGES UNDER THE NDPS ACT (NO.61 OF 1985) POSTED IN SANGRUR SESSIONS DIVISION (as on 09 August 2021) Sr. No. Name of Judicial Officer Designation Name of Police Station 1 Ms. Baljinder Siddhu ASJ City Sangrur, GRP Sangrur and City-1 Sangrur 2 Sh. Baljinder Singh-II ASJ Longowal and Sadar Sangrur 3 Sh. Sham Lal ASJ City / Sadar Sunam, Moonak and Dirba 4 Ms. Saru Mehta Kaushik ASJ Cheema, Khanauri, Bhawanigarh and Chajli City Dhuri, City / Sadar Ahmedgarh, Sadar 5 Ms. Smriti Dhir ASJ Dhuri and Sherpur City / Sadar Amargarh, Sandaur,Dharmgarh and 6 Ms. Girish ASJ City Malerkotla-2 7 Sh. Gurpartap Singh ASJ City-1 Malerkotla and Lehra LIST OF POLICE STATIONS ALLOTED TO THE JUDICIAL MAGISTRATES POSTED IN SANGRUR SESSIONS DIVISION Sr. No. Name of Judicial Officer Designation Name of Police Station Sadar Sangrur, Police Post Channo, Bhawanigarh 1 Sh. Ajit Pal Singh-II CJ(SD) and Police Post Gharachon City Sangrur,Vigilance Bureau Patiala, RPF, 2 Sh. Harvinder Singh Sindhia CJM GRP and NRI Sangrur 3 Sh. Rahul Kumar ACJ(SD) Longowal and Police Post Badrukhan 4 Sh. Amrish Kumar Jain CJ(JD) Exclusive Court 138 NI ACT and City 1 Sangrur DHURI Sr. No. Name of Judicial Officer Designation Name of Police Station 1 Ms. Neha Goel ACJ(SD) City Dhuri and GRP 2 Ms. Lavleen Sandhu CJ(JD) Sherpur and Sadar Dhuri SUNAM Sr. No. Name of Judicial Officer Designation Name of Police Station 1 Ms. Amandeep Kaur-I ACJ(SD) City / Sadar Sunam and Cheema Ms. -

Roaster for Petrol Pumps of District Sangrur for the Period Between 15.04.2020 to 30.04.2020 Date Name of Petrol Pump Address Contact No
Roaster for Petrol Pumps of District Sangrur for the period between 15.04.2020 to 30.04.2020 Date Name of Petrol Pump Address Contact No. 15/4/2020 Prem Filling Station Sangrur 9216155460 15/4/2020 Parmeshwar Oil Co. Jarg Chowk, Malerkotla 9417252597 15/4/2020 DIRBA FILLING STATION Patran Road, Dirba 9814926380 15/4/2020 KRISHNA PETROL PUMP BADBAR ROAD 9464221316 15/4/2020 TILAK RAJ HANS RAJ FILLING STATION SULLARGHARAT 9876045123 15/4/2020 Bharpur Filling Station Sunam Road, Phaguwala 9463774397 15/4/2020 vardhman filling station patran road moonak 9465357625 15/4/2020 Satnam Filling Station Khanouri 9876457250 15/4/2020 SUNAM HP CENTER Sunam 8822477777 15/4/2020 SINGLA FILLING STATION Ahmedgarh 9815770825 15/4/2020 RIL LEHAL KHURD lehragaga 15/4/2020 KISSAN SERVICE CENTRE (BENRA) DHURI 9855047015 15/4/2020 FAIR DEAL FILLING STATION CHEEMA 9417655399 15/4/2020 SUPER FUEL CENTRE NABHA- MALERKOTLA ROAD, VILLAGE BAGRIAN 9815360269 15/4/2020 Malwa Filling Station Shergarh Cheema 6239404828 16/4/2020 Pal Filling Station Sangrur 9855447771 16/4/2020 Pipli Filling Station Pipli Chowk, Malerkotla 9814920959 16/4/2020 HAMARA PUMP, DIRBA Kaimpur Road, Dirba 9815993528 16/4/2020 KL FUEL CENTRE SHERON ROAD 9877379180 16/4/2020 SINGLA FILLING STATION SULLARGHARAT 9872416371 16/4/2020 MITTAL FILLING STATION Patiala Road, Bhawanigarh 9356284590 16/4/2020 jai bharat oil patran road moonak 9417542464 16/4/2020 Satnam Filling Station Khanouri 9876457250 16/4/2020 CITY PETROLEUMS Sunam 9815343954 16/4/2020 Super Filling Station Ahmedgarh 9809510000 16/4/2020 -
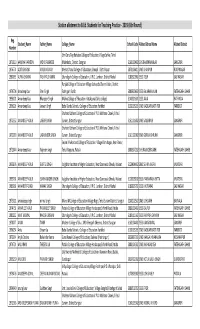
Station Allotment to B.ED. Teaching Practice
Station allotment to B.Ed. Students for Teaching Practice - 2019 (6th Round) Reg. Student_Name Father_Name Collage_Name School Code Alloted School Name Alloted District Number Shri Guru Teg Bahadur College of Education,Village Sehke, Tehsil 1970310 AAMINA PARVEEN MOHD RASHEED Malerkotla, District. Sangrur 3160100402 GHS BAURHAI KALAN SANGRUR 1976774 ADITI SHARMA VINOD KUMAR Mehar Chand College of Education,Bhnopli . Distt. Ropar 3070106411 GMS KHANPUR ROOPNAGAR 1980095 ALPNA SHARMA YASHPAL SHARMA Chandigarh College of Education, V.P.O. Landran, District Mohali 3180310902 GSSS TEUR SAS NAGAR Punjab College of Education Village Sarkapda Chunni Kalan, District 1974704 Amadeep Kaur Sher Singh Fatehgarh Sahib 3080300602 GSSS BALARHI KALAN FATEHGARH SAHIB 1996121 Amandeep Kaur Bharpoor Singh Malwa College of Education. Kotakpura (Girls college) 3140201601 GSSS JALAL BATHINDA 1996226 Amandeep Kaur Jeewan Singh Baba Banda Bahadur College of Education. faridkot 3130105203 GMS BAZIGAR BASTI FDK FARIDKOT Shaheed Udham College of Education V.P.O. Mohlana Chowk, Tehsil 1972352 AMANDEEP KAUR JAGSIR SINGH Sunam, District Sangrur 3161101802 GMS SALEMPUR SANGRUR Shaheed Udham College of Education V.P.O. Mohlana Chowk, Tehsil 1972030 AMANDEEP KAUR JAGMINDER SINGH Sunam, District Sangrur 3161102902 GMS GURBAKSHPURA SANGRUR Swami Vivekanand College of Education. Village Ram Nagar, Near Banur, 1972844 Amandeep Kaur Rajinder singh Tehsil Rajpura, Patiala 3080307202 GHS RAJINDERGARH FATEHGARH SAHIB 1993876 AMANDEEP KAUR GURTEJ SINGH Kalgidhar Institute of Higher Education, Near Danewala Chwok, Malout 3120404402 GMS SEHNA KHERA MUKTSAR 1995761 AMANDEEP KAUR SUKHMANDER SINGH Kalgidhar Institute of Higher Education, Near Danewala Chwok, Malout 3120203102 GSSS PANNIWALA FATTA MUKTSAR 1980261 AMANDEEP SINGH NANAK SINGH Chandigarh College of Education, V.P.O. Landran, District Mohali 3180305702 GSSS KHIZRABAD SAS NAGAR 1973011 amandeep singh nirmal singh M and M College of Education Village Nagri,Tehsil Sunam District. -

Sangrur District Punjab
SANGRUR DISTRICT PUNJAB CENTRAL GROUND WATER BOARD Ministry of Water Resources Government of India North Western Region CHANDIGARH 2013 Contributors Tejdeep Singh Scientist ‘C’ Prepared under supervision of A.K.Bhatia Regional Director Our Vision “Water Security through Ground water Management” GROUND WATER INFORMATION BOOKLET SANGRUR DISTRICT, PUNJAB CONTENTS SANGRUR DISTRICT AT A GLANCE 1.0 INTRODUCTION 2.0 RAINFALL AND CLIMATE 3.0 GEOMORPHOLOGY AND SOILS 4.0 GROUND WATER SCENARIO 4.1 HYDROGEOLOGY 4.2 GROUND WATER RESOURCES 4.3 GROUND WATER QUALITY 4.4 STATUS OF GROUND WATER DEVELOPMENT 5.0 GROUND WATER MANAGEMENT STRATEGY 5.1 GROUND WATER DEVELOPMENT 5.2 WATER CONSERVATION AND ARTIFICIAL RECHARGE 6.0 GROUND WATER RELATED ISSUES & PROBLEMS 7.0 AWARENESS AND TRAINING ACTIVITY 8.0 AREA NOTIFIED BY CGWA 9.0 RECOMMENDATIONS SANGRUR DISTRICT AT A GLANCE S. No ITEMS STATISTICS 1 GENERAL INFORMATION i) Geographical area (sq.km) 5020Sq.Km ii) Administrative Divisions (as on 31.03.05) Number of Block 9. Ahmedgarh, Sangrur, Bhawanigarh, Malerkotla Dhuri Sherpur, , Sunam Lehragage, Andana - iii)Population (as on 2011 Census) 1654408 iv) Normal Annual Rainfall (mm) 552 2. GEOMORPHOLOGY Major Physiographic units Sirhind Canal Major Drainage Ghaggar river 3. LAND USE (Sq.Km) a) Forest area 70 b)Net area shown 4400 c) cultivable area --4440 4. MAJOR SOIL TYPES 5. AREA UNDER PRINCIPAL CROPS (Sq.Km) 2020 rice 2270 wheat Rabi Crops-940 6. IRRIGATION BY DIFFERENT SOURCES (Areas and Numbers of Structures) Tube Wells 4110 km2/ 1,27236 Canals 290 km2/ Sirhind canal & its tributaries Other Sources - Net Irrigated area 4400 km2 Gross irrigated area 8710 km2 7. -

D:\Diary 2020\Dairy New 2020 N
1 Name & Designation Phone Residence Off. Resi. Address gzikp oki GtB PUNJAB RAJ BHAWAN thagha f;zx pdB"o, okigkb 2740740 2740608 Punjab Raj V.P. Singh Badnore, Governor 2746116 2740681 Bhawan/6 wdB gkb, ;eZso$okigkb 2740608 2685090 244/55 Madan Pal, Secy. to Governor 99146-00844 Chd. i/a n?wa pkbkw[o[rB, gqw[Zy ;eZso$okigkb 2740592 2746033 58/5 J. M. Balamurugan, Prin.Secy.to Governor 97800-20243 Chd. r[bôB e[wko, fBZih ;eZso$;eZso$okigkb 2740608 98780-45680 478-A, Gulshan Kumar, Pvt. Secy./Secy. to Governor Harmilap Nagar, Baltana e/apha f;zx, vhankJhaiha J/avha;ha (gh) okigkb 2740609 2971802 31/7-A K.B. Singh, DIG, ADC(P) Governor 98725-21114 w/io g[ôg/Adok f;zx, J/avha;ha (n?w)$okigkb 2740696 94604-30543 52/7-A Maj. Pushpendra Singh, ADC(M)/Governor fôyk Bfjok (ôqhwsh), nkJhaghHnkoHUa 2746095 2773319 2237/ whvhnk okigkb 97800-36106 15-C,Chd. Shikha Nehra (Mrs.), IPRO (Media) to Governor vkH nwohe f;zx uhwk, n?wHTH$ 2792597 2632955 3379/ nkoHphH fv;g?A;oh 97799-13379 46-C Dr. Amrik Singh Cheema, MO, R.B. Disp. Chd. i;d/t f;zx f;ZX{, n?;a gha ;[oZfynk 2740482 98763-71155 1122/69 Jasdev Singh Sidhu, SP Security EPABX-2743224, 2740602, 2740608-10, 2740681, Fax : 2741058 g³ikp ftXkB ;Gk PUNJAB VIDHAN SABHA okDk e/a gha f;zx, ;gheo 2740372 2742976 10/2 Rana K.P. Singh, Speaker 2740739 2740842 F-2740473 okw b'e yskBk, ;eZso$;gheo 2740372 80542-00024 1605/ Ram Lok Khatana, Secretary to Speaker 2740739 94784-44433 38-B okfizdo gq;kd, ftô/ô ekoi nc;o$;gheo 2740372 98722-23329 290/7 Rajinder Prasad, OSD to Speaker ;[fozdo f;zx w'sh, fBZih ;eZso$;gheo 2740739 80543-00021 999/3B-2 Surinder Singh Moti, Pvt. -
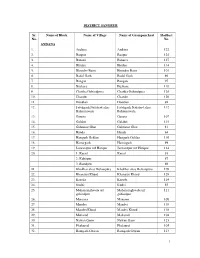
DISTRICT SANGRUR Sr. No. Name of Block Name of Village Name Of
DISTRICT SANGRUR Sr. Name of Block Name of Village Name of Grampanchyat Hadbast No. No. ANDANA 1. Andana Andana 122 2. Baopur Baopur 125 3. Banarsi Banarsi 127 4. Bhulan Bhulan 114 5. Bhunder Baini Bhunder Baini 101 6. Badal Garh Badal Garh 96 7. Bangan Bangan 97 8. Bushara Bushara 119 9. Chattha Gobindpura Chattha Gobindpura 126 10. Chandu Chandu 120 11. Doodian Doodian 94 12. Fatehgarh Nauabad alias Fatehgarh Nauabad alias 112 Bahminiwala Bahminiwala 13. Ganeta Ganeta 107 14. Galahri Galahri 115 15. Gahmoor Ghat Gahmoor Ghat 81 16. Handa Handa 84 17. Harigarh Gehlan Harigarh Gehlan 110 18. Hamirgarh Hamirgarh 99 19. Jaswantpur urf Hotipur Jaswantpur urf Hotipur 124 20. 1. Karail Karail 83 2. Kabirpur 87 3. Bazidpur 89 21. Khokhar alias Bishanpura Khokhar alias Bishanpura 109 22. Khanauri Khurd Khanauri Khurd 128 23. Karoda Karoda 116 24. Kudni Kudni 85 25. Mahansinghwala urf Mahansinghwala urf 111 gobindpur gobindpur 26. Maniana Maniana 108 27. Mandvi Mandvi 118 28. Mandvi Khurd Mandvi Khurd 118 29. Makarod Makarod 104 30. Nawan Gaon Nawan Gaon 123 31. Phalaund Phalaund 105 32. Ramgarh Gujran Ramgarh Gujran 113 1 Sr. Name of Block Name of Village Name of Grampanchyat Hadbast No. No. 33. Rampur Gujran Rampur Gujran 86 34. Rajal Heri Rajal Heri 98 35. Shanpur Nauabad alias Thir Shanpur Nauabad alias Thir 121 36. Salimgarh Salimgarh 102 37. Shergarh Shergarh 95 38. Surjan Bhaini Surjan Bhaini 103 39. Thaska Thaska 117 BHAWANIGARH 1. 1. Aloarkh Aloarkh 38 2. Panjbiri 39 2. Akbarpur Akbarpur 103 3. Bakhtara Bakhtara 17 4. Bakhtari Bakhtari 18 5. -

Travel Agents Who Have Been Issued License by the Office of District Magistrate
Travel Agents who have been issued license by the office of District Magistrate. S. License Number Name and address of Travel Name of Firm and Date of Issue of License and License Type Contact No. No. agent address Valid upto 1 01/DC/MA/SGR/2018 Sh. Arun Jindal S/o Sohan Lal Jindal Travels Guru Teg 01.04.2016 to 31.03.2021. Travel Agency 98761-22687 Jindal, House No. 215-2 ward No. Bahadur Nagar Kakarwal Note- Cancelled on account of 8A, GTB Nagar, Dhuri. Chowk, Sangrur Road, address by the applicant and Dhuri. New License has been issued Note- Cancelled on to the Applicant i.e. account of address by the 03/DC/MA/SGR/2018. applicant and New License has been issued to the Applicant i.e. 03/DC/MA/SGR/2018. 2 02/DC/MA/SGR/2018 Sh. Gurpreet Singh S/o G4 Seven Immigration 15.01.2018 to 14.01.2023 Immigration Sukhwinder Singh, VPO Mehlan, Consultancy Thales Bagh Consultancy District Sangrur Colony, Nabha Gate Sangrur. 3 03/DC/MA/SGR/2018 Sh. Arun Jindal S/o Sohan Lal Jindal Travels Guru Teg 01.04.2016 to 31.03.2021 Travel Agency 98761-22687 Jindal, House No. 215-2 ward No. Bahadur Nagar Kakarwal 8A, GTB Nagar, Dhuri. Chowk, Sangrur Road, Dhuri. 4 04/DC/MA/SGR/2018 Sh. Sukhwinder Singh S/o Sh. Jang TEHDIL, A point of 15.03.2018 To 14.03.2023 Immigration 9888329022 Singh R/o House No. 40, Gali No. English Language Opp. Consultancy 4, Shivam Colony, Nankiyana Kaula Park, Main Road, Chowk, Sangrur. -

Whoswho 2018.Pdf
Hindi version of the Publication is also available सत㔯मेव ज㔯ते PARLIAMENT OF INDIA RAJYA SABHA WHO’S WHO 2018 (Corrected upto November 2018) सं द, र ी㔯 स ाज㔯 त सभ ार ा भ P A A H R B LI A AM S EN JYA T OF INDIA, RA RAJYA SABHA SECRETARIAT NEW DELHI © RAJYA SABHA SECRETARIAT, NEW DELHI http://parliamentofindia.nic.in http://rajyasabha.nic.in E-mail: [email protected] EDITORIAL TEAM Shri D. S. Prasanna Kumar Director Smt. Vandana Singh Additional Director Smt. Asha Singh Joint Director Shri Md. Salim Ali Assistant Research Officer Shri Mohammad Saleem Assistant Research Officer PUBLISHED BY THE SECRETARY-GENERAL, RAJYA SABHA AND PRINTED BY DRV GrafIx Print, 41, Shikshak BHAwAN, INSTITUTIonal AREA, D-BLoCK JANAKPURI, NEw DELHI-110058 PREFACE TO THE THIRTY-FOURTH EDITION Rajya Sabha Secretariat has the pleasure of publishing the updated thirty- fourth edition of ‘Rajya Sabha who’s who’. Subsequent to the retirement of Members and biennial elections and bye- elections held in 2017 and 2018, information regarding bio-data of Members have been updated. The bio-data of the Members have been prepared on the basis of information received from them and edited to keep them within three pages as per the direction of the Hon’ble Chairman, Rajya Sabha, which was published in Bulletin Part-II dated May 28, 2009. The complete bio-data of all the Members are also available on the Internet at http://parliamentofindia.nic.in and http://rajyasabha.nic.in. NEW DELHI; DESH DEEPAK VERMA, December, 2018 Secretary-General, Rajya Sabha CONTENTS PAGES 1. -

Water Table Behaviour in Punjab: Issues and Policy Options
WATER TABLE BEHAVIOUR IN PUNJAB: ISSUES AND POLICY OPTIONS Karam Singh* Abstract Punjab faces a sever problem of declining water tables by as much as 10 – 15m in most parts. The paper focuses on groundwater behavior in various parts (Blocks) of the Punjab in categories of low to high rainfall regions, saline to sweet groundwater zones, scanty to extensive canal water supply areas, the uplands to riverbeds and the cropping pattern in terms of low to high water intensive crops. Any changes in these parameters will affect the recharge and withdrawal of groundwater. In was found that as the area under rice cultivation increased, there was a corresponding decline in ground water recharge. It is often advocated that pricing policy for wheat and rice (Minimum Support Price (MSP) and its effectiveness) and free electricity supply are responsible for the critical ground water situation in Punjab. The paper tires to examine this and look at policy measures needed to address the situation. 1. INTRODUCTION The groundwater situation in Punjab has been a serious issue for a long time now. The total water requirement for Punjab, with the present cropping pattern and practices and industrial uses, is estimated at 4.33 million ha metres. It varies from 4.30 to 4.40 million ha metres. The total availability of water is estimated at 3.13 million ha metres out of which 1.45 million ha meters is from canals and 1.68 ha meters is from rainfall and seepage. The deficit of almost 1.20 million ha metres is met by ground water withdrawal. -
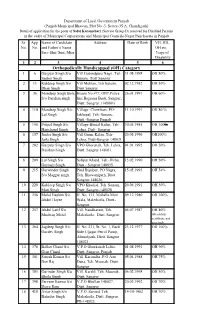
Safai Karamchari Sangrur.Pdf
Department of Local Government Punjab (Punjab Municipal Bhawan, Plot No.-3, Sector-35 A, Chandigarh) Detail of application for the posts of Safai Karamchari (Service Group-D) reserved for Disabled Persons in the cadre of Municipal Corporations and Municipal Councils-Nagar Panchayats in Punjab Sr App Name of Candidate Address Date of Birth VH, HH, No. No. and Father’s Name OH etc. Sarv Shri/ Smt./Miss %age of Disability 1 2 3 4 5 6 Orthopedically Handicapped (OH) Category 1 6 Gurpyar Singh S/o Vill Gobindpura Nagri, Teh 31.08.1998 OH 80% Saeber Singh Sunam, Distt Sangrur 2 11 Kuldeep Singh S/o Vill Mehlan, Teh Sunam, 02.12.1982 OH 50% Dhan Singh Distt Sangrur 3 36 Mandeep Singh Bola House No #77, OPP.Police 26.01.1991 OH 60% S/o Darshan singh line Baguana Basti, Sangrur, Distt: Sangrur. (148001) 4 114 Mandeep Singh S/o Village- Chowbass, PO- 11.10.1991 OH 80 % Lal Singh Jakhepal, Teh.-Sunam, Distt.-Sangrur Punjab 5 146 Pritpal Singh S/o Village-Bhutal Kalan, Teh- 30.05.1985 OH 100% Harichand Singh Lehra, Distt- Sangrur 6 157 Jinder Singh S/o Vill.Gurne Kalan, Teh- 20.05.1996 OH100% Jaila Singh Lehra, Distt-Sangrur 148031 7 202 Gurpyar Singh S/o VPO Ghorenab, Teh. Lehra, 01.01.1992 OH 50% Darshan Singh Distt. Sangrur 148031. 8 209 Lal Singh S/o Safipur Khurd, Teh.-Dirba, 15.02.1990 OH 50% Gurmail Singh Distt.- Sangrur 148035. 9 215 Gurwinder Singh Pind Bijalpur, PO Nagra, 15.05.1993 OH 54% S/o Maggar singh Teh.