Sedar50-Rd26
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (Ogrefish) by J.A
click for previous page 1178 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (ogrefish) by J.A. Moore, Florida Atlantic University, USA iagnostic characters: Small (to about 160 mm standard length) beryciform fishes.Body short, deep, and Dcompressed, tapering to narrow peduncle. Head large (1/3 standard length). Eye smaller than snout length in adults, but larger than snout length in juveniles. Mouth very large and oblique, jaws extend be- hind eye in adults; 1 supramaxilla. Bands of villiform teeth in juveniles are replaced with large fangs on dentary and premaxilla in adults; vomer and palatines toothless. Deep sensory canals separated by ser- rated ridges; very large parietal and preopercular spines in juveniles of one species, all disappearing with age. Gill rakers as clusters of teeth on gill arch in adults (lath-like in juveniles). No true fin spines; single, long-based dorsal fin with 16 to 20 rays; anal fin very short-based with 7 to 9 soft rays; caudal fin emarginate; pectoral fins with 13 to 16 soft rays; pelvic fins with 7 soft rays. Scales small, non-overlapping, spinose, goblet-shaped in adults; lateral line an open groove partially bridged by scales; no enlarged ventral keel scutes. Colour: entirely dark brown or black in adults. Habitat, biology, and fisheries: Meso- to bathypelagic, at depths of 75 to 5 000 m. Carnivores, with juveniles feeding on mainly crustaceans and adults mainly on fishes. May sometimes swim in small groups. Uncommon deep-sea fishes of no commercial importance. Remarks: The family was revised recently by Kotlyar (1986) and contains 1 genus with 2 species throughout the tropical and temperate latitudes. -

Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA
ACTA ICHTHYOLOGICA ET PISCATORIA (2016) 46 (4): 287–291 DOI: 10.3750/AIP2016.46.4.02 MICROSATELLITE DNA-BASED GENETIC TRACEABILITY OF TWO POPULATIONS OF SPLENDID ALFONSINO, BERYX SPLENDENS (ACTINOPTERYGII: BERYCIFORMES: BERYCIDAE)—PROJECT CELFISH—PART 2 Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA Division of Aquaculture, West Pomeranian University of Technology, Szczecin, Kazimierza Krolewicza 4, 71-550 Szczecin, Poland Kempter J., Kiełpinski M., Panicz R., Keszka S. 2016. Microsatellite DNA-based genetic traceability of two populations of splendid alfonsino, Beryx splendens (Actinopterygii: Beryciformes: Berycidae)— Project CELFISH—Part 2. Acta Ichthyol. Piscat. 46 (4): 287–291. Background. The study is a contribution to Project CELFISH which involves genetic identifi cation of populations of fi sh species presenting a particular economic importance or having a potential to be used in the so-called commercial substitutions. The EU fi sh trade has been showing a distinct trend of more and more fi sh species previously unknown to consumers being placed on the market. Molecular assays have become the only way with which to verify the reliability of exporters. This paper is aimed at pinpointing genetic markers with which to label and differentiate between two populations of splendid alfonsino, Beryx splendens Lowe, 1834, a species highly attractive to consumers in Asia and Oceania due to the meat taste and low fat content. Material and methods. DNA was isolated from fragments of fi ns collected at local markets in Japan (MJ) (n = 10) and New Zealand (MNZ) (n = 18). The rhodopsin gene (RH1) fragment and 16 microsatellite DNA fragments (SSR) were analysed in all the individuals. -

Order BERYCIFORMES ANOPLOGASTRIDAE Anoplogaster
click for previous page 2210 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths by J.R. Paxton iagnostic characters: Small (to 16 cm) Dberyciform fishes, body short, deep, and compressed. Head large, steep; deep mu- cous cavities on top of head separated by serrated crests; very large temporal and pre- opercular spines and smaller orbital (frontal) spine in juveniles of one species, all disap- pearing with age. Eyes smaller than snout length in adults (but larger than snout length in juveniles). Mouth very large, jaws extending far behind eye in adults; one supramaxilla. Teeth as large fangs in pre- maxilla and dentary; vomer and palatine toothless. Gill rakers as gill teeth in adults (elongate, lath-like in juveniles). No fin spines; dorsal fin long based, roughly in middle of body, with 16 to 20 rays; anal fin short-based, far posterior, with 7 to 9 rays; pelvic fin abdominal in juveniles, becoming subthoracic with age, with 7 rays; pectoral fin with 13 to 16 rays. Scales small, non-overlap- ping, spinose, cup-shaped in adults; lateral line an open groove partly covered by scales. No light organs. Total vertebrae 25 to 28. Colour: brown-black in adults. Habitat, biology, and fisheries: Meso- and bathypelagic. Distinctive caulolepis juvenile stage, with greatly enlarged head spines in one species. Feeding mode as carnivores on crustaceans as juveniles and on fishes as adults. Rare deepsea fishes of no commercial importance. Remarks: One genus with 2 species throughout the world ocean in tropical and temperate latitudes. The family was revised by Kotlyar (1986). Similar families occurring in the area Diretmidae: No fangs, jaw teeth small, in bands; anal fin with 18 to 24 rays. -
![Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 South East](https://docslib.b-cdn.net/cover/1518/stock-status-report-alfonsino-bys-doc-sc-11-2019-south-east-341518.webp)
Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 South East
Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 STATUS REPORT Beryx splendens Alfonsino FAO -ASFIS code: BYS 2019 Updated 21 November 2019 South East Atlantic Fisheries Organization [SEAFO] 1 Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 TABLE OF CONTENTS 1. Description of the fishery ....................................................................................................................... 3 1.1 Description of fishing vessels and fishing gear .......................................................................... 3 1.2 Spatial and temporal distribution of fishing ............................................................................... 6 1.3 Reported retained catches and discards ..................................................................................... 9 1.4 IUU catch ............................................................................................................................... 12 2. Stock distribution and identity ............................................................................................................. 12 3. Data available for assessments, life history parameters and other population information ..................... 12 3.1 Fisheries and surveys data ....................................................................................................... 12 3.2 Length data and frequency distribution ................................................................................... 13 3.3 Length-weight relationships ................................................................................................... -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -
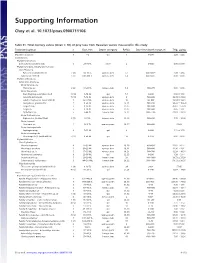
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Atlas Over Danske Saltvandsfisk Nordisk Beryx
Atlas over danske saltvandsfisk Nordisk beryx Beryx decadactylus Cuvier, 1829 Af Henrik Carl Nordisk beryx på 48,5 cm fanget på Bøchers Banke, 17. november 2010 © Henrik Carl. Projektet er finansieret af Aage V. Jensen Naturfond Alle rettigheder forbeholdes. Det er tilladt at gengive korte stykker af teksten med tydelig kildehenvisning. Teksten bedes citeret således: Carl, H. 2019. Atlas over danske saltvandsfisk – Nordisk beryx. Statens Naturhistoriske Museum. Online-udgivelse, februar 2019. Systematik og navngivning Der er gennem tiden beskrevet ti arter i slægten Beryx Cuvier, 1829. Tre af disse er siden flyttet til familiens anden slægt Centroberyx, og fire arter har vist sig at være synonymer af nordisk beryx. Slægten består nu således af tre arter, hvoraf de to findes i europæiske farvande. Foruden nordisk beryx drejer det sig om pragt-beryx, Beryx splendens. Sidstnævnte kendes ikke fra dansk farvand, men blev fanget ved Sverige i 2011 (Jonsson 2012). Ynglen har tidligere været beskrevet under slægtsnavnet Actinoberyx Roule, 1923. Det officielle navn er nordisk beryx – et navn, der er blevet brugt siden midt i 1900-tallet (Bruun & Pfaff 1950). Før den tid havde fisken ikke noget dansk navn. Slægtsnavnet Beryx stammer fra det antikke Grækenland, hvor det var navnet på en ukendt fiskeart. Artsnavnet decadactylus betyder tifingret, hvilket hentyder til, at arten normalt har ti bugfinnestråler (Kullander & Delling 2012). Udseende og kendetegn Kroppen er høj og sammentrykt. Den største højde udgør 44-50 % af standardlængden (længden fra snuden til basis af halefinnen) (Maul 1986). Hovedet er stort, og munden er ligeledes stor med et tydeligt underbid. Munden kan spiles vidt op. -

Beryx Splendens Lowe, 1834
Beryx splendens Lowe, 1834 AphiaID: 126395 SPLENDID ALFONSINO Animalia (Reino) > Chordata (Filo) > Vertebrata (Subfilo) > Gnathostomata (Infrafilo) > Pisces (Superclasse) > Pisces (Superclasse-2) > Actinopterygii (Classe) > Beryciformes (Ordem) > Berycidae (Familia) Heessen, Henk Sinónimos Bryx splendens Lowe, 1834 Referências MARTINS, R.; CARNEIRO, M., 2018. Manual de identificação de peixes ósseos da costa continental portuguesa – Principais Características Diagnosticantes. IPMA, I.P., 204p Fernandes, P., Collette, B., Heessen, H., Smith-Vaniz, W.F. & Herrera, J. 2015. Beryx splendens. The IUCN Red List of Threatened Species 2015: e.T16425354A45791585. Downloaded on 05 August 2019. additional source Froese, R. & D. Pauly (Editors). (2018). FishBase. World Wide Web electronic publication. , available online at http://www.fishbase.org [details] basis of record van der Land, J.; Costello, M.J.; Zavodnik, D.; Santos, R.S.; Porteiro, F.M.; Bailly, N.; 1 Eschmeyer, W.N.; Froese, R. (2001). Pisces, in: Costello, M.J. et al. (Ed.) (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels, 50: pp. 357-374 [details] additional source Gulf of Maine Biogeographic Information System (GMBIS) Electronic Atlas. 2002. November, 2002. [details] additional source Hareide, N.R. and G. Garnes. 1998. The distribution and abundance of deep water fish along the Mid-Atlantic Ridge from 43°N to 61°N. Theme session on deep water fish and fisheries. ICES CM 1998/O:39. [details] additional source Welshman, D., S. Kohler, J. Black and L. Van Guelpen. 2003. An atlas of distributions of Canadian Atlantic fishes. , available online at http://epe.lac-bac.gc.ca/100/205/301/ic/cdc/FishAtlas/default.htm [details] additional source Streftaris, N.; Zenetos, A.; Papathanassiou, E. -

Title World Distribution of the Baloon Alfonsin, Beryx Mollis
World distribution of the Baloon Alfonsin, Beryx mollis Title (Pisces: Beryciformes: Berycidae) Author(s) Yoshino, Tetsuo; Kotlyar, Alexandor N. 琉球大学理学部紀要 = Bulletin of the College of Science. Citation University of the Ryukyus(72): 119-123 Issue Date 2001-09 URL http://hdl.handle.net/20.500.12000/15192 Rights Bull. Fac. Sci., Univ. Ryukyus, No.73 : 63 (2002) 63 Errata: World distribution of the Baloon Alfonsin, Beryx mollis (Pisces: Beryciformes: Berycidae) [Bull. Fac. Sci., Univ. Ryukyus, 72: 119-123 (2001)] Tetsuo Yoshino and Alexandor N. Kotlyar The following misprints in above published paper should be corrected. The title of Table 1 was missing. Table 1. Selected counts and proportional measurements expressed as percentages of standard length of Beryx mollis Source Present study Yoshino et al. (1999) Locality Socotra Error Equator South China Southern Seamount Seamount Sea Japan No. of specimens 3 67 1 2 21 Dorsal fin IV, 12-13 IV, 12-13 IV, 12 IV. 12 IV, 12-13 Anal fin IV. 25-27 IV-V, 24-28 IV, 25 IV. 28 IV, 27-32 Pectoral fin 16 15-17 15 15-16 16-18 Pelvic fin I, 10 I, 10-11 I, 10 I, 9-10 I, 9-10 Gill rakers 7+18-19 6-7+16-20 7 + 17 6+17 6-7 + 14-17 Lateral line scales 66-74 64-79 73 68-71 60-69 Pyloric caeca 13-17 12-20* 18 15" 15-20 Standard length (mm) 144-177 124-243 305 144-225 114-308 Body depth 38.0-41.1 34.6-41.8 39.1 38.2-44.0 39.0-43.2 Head length 35.0-35.6 32.8-38.0 35.4 31.3-35.1 31.8-39.3 Eye diameter 13.7-14.2 12.6-17.3 15.7 13.5-15.1 13.8-15.9 Interorbital width 7.1-7.9 5.1-8.5 7.9 7.6-8.2 6.8-8.4 Maxillary length 18.8-19.2 16.9-20.7 20.0 17.4-18.7 17.1-19.5 Caudal peduncle depth 10.3-11.0 8.2-11.0 10.8 9.4-10.2 6.7-10.4 Caudal peduncle length 12.5-15.1 10.9-15.2 12.8 10.9-11.5 11.1-14.3 Pectoral fin length 24.3-28.8 23.3-30.0 28.2 26.0" 25.1-31.8 Pelvic fin length 22.2-24.3 19.4-27.8 25.9 24.0-25.1 24.1-33.2 'based on 38 specimens "based on one specimen Bull. -

SEAFO SCR Doc 01/2009 SPECIES PROFILE PROPOSAL for THE
SEAFO SCR Doc 01/2009 SPECIES PROFILE PROPOSAL FOR THE SCIENTIFIC BODIES OF SEAFO L.J. López-Abellán (1) , I. Figueiredo (2) and R. Sarralde (1) (1) Centro Oceanográfico de Canarias, Instituto Español de Oceanografía, Avda. Tres de Mayo nº 73, 38005 Santa Cruz de Tenerife, España; (2) Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB),Av. Brasília, 1449-006 Lisboa, Portugal This paper has been presented for consideration by SEAFO and may contain unpublished data, analyses, and/or conclusions subject to change. Data in this paper shall not be cited or used for purposes other than the work of the SEAFO Commission, Scientific Committee or their subsidiary bodies without the permission of the originators and/or owners of the data. SPECIES PROFILE PROPOSAL FOR THE SCIENTIFIC BODIES OF SEAFO L.J. López-Abellán (1) , I. Figueiredo (2) and R. Sarralde (1) (1) Centro Oceanográfico de Canarias, Instituto Español de Oceanografía, Avda. Tres de Mayo nº 73, 38005 Santa Cruz de Tenerife, España; (2) Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB),Av. Brasília, 1449-006 Lisboa, Portugal BACKGROUND Some regional organisations similar to SEAFO have promoted and adopted the creation of templates for compiling and summarising the best information about fisheries and species within their management areas (e.g. CCAMLR, SPRFMO). The aim of this species profile is to compile in a living document the current best available information about: i) the biology, ecology and population dynamics of the main species; ii) fisheries data; iii) facts or events affecting both the species and their environment; and iv) the evolution of their fisheries in the regional management area. -

Davide DI BLASI1*, Erica CARLIG1, Sara FERRANDO2, Laura GHIGLIOTTI1, Peter N
ACTA ICHTHYOLOGICA ET PISCATORIA (2018) 48 (2): 183–188 DOI: 10.3750/AIEP/02340 A NEW RECORD AND BIOLOGICAL EVIDENCE SUPPORTING THE ESTABLISHMENT OF BERYX SPLENDENS (ACTINOPTERYGII: BERYCIFORMES: BERYCIDAE) IN THE WESTERN MEDITERRANEAN BASIN Davide DI BLASI1*, Erica CARLIG1, Sara FERRANDO2, Laura GHIGLIOTTI1, Peter N. PSOMADAKIS3, and Marino VACCHI1 1 Institute of Marine Sciences (ISMAR), National Research Council (CNR), Genoa, Italy 2 Department of Earth, Environmental and Life Sciences (DISTAV), University of Genoa, Genoa, Italy 3 Food and Agriculture Organization of the United Nations (FAO), Rome, Italy Di Blasi D., Carlig E., Ferrando S., Ghigliotti L., Psomadakis P.N., Vacchi M. 2018. A new record and biological evidence supporting the establishment of Beryx splendens (Actinopterygii: Beryciformes: Berycidae) in the western Mediterranean basin. Acta Ichthyol. Piscat. 48 (2): 183–188. Abstract. A new specimen of splendid alfonsino, Beryx splendens Lowe, 1834, was caught by trawling in July 2016 in the Ligurian Sea at the depth of 350 m, in the proximity of a submarine canyon. It represents the 10th documented record of B. splendens in the Mediterranean. Because of the rarity of the records in the basin, there could be doubts whether to consider or not such species as established in the area. However, some findings may support the hypothesis of the possible establishment of the species in the Mediterranean Sea. For example, the stomach of the specimen was nearly full, with rests of one crustacean decapod, one fish, and some cephalopods, which are commonly reported prey items for the species. Macroscopic observation and histological analysis of the gonads revealed that the specimen was a male in an advanced stage of gonadal development. -

Commented Checklist of Marine Fishes from the Galicia Bank Seamount (NW Spain)
Zootaxa 4067 (3): 293–333 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.4067.3.2 http://zoobank.org/urn:lsid:zoobank.org:pub:50B7E074-F212-4193-BFB9-84A1D0A0E03C Commented checklist of marine fishes from the Galicia Bank seamount (NW Spain) RAFAEL BAÑON1,2, JUAN CARLOS ARRONTE3, CRISTINA RODRIGUEZ-CABELLO3, CARMEN-GLORIA PIÑEIRO4, ANTONIO PUNZON3 & ALBERTO SERRANO3 1Servizo de Planificación, Dirección Xeral de Recursos Mariños, Consellería de Pesca e Asuntos Marítimos, Rúa do Valiño 63–65, 15703 Santiago de Compostela, Spain. E-mail: [email protected] 2Grupo de Estudos do Medio Mariño (GEMM), puerto deportivo s/n 15960 Ribeira, A Coruña, Spain 3Instituto Español de Oceanografía, C.O. de Santander, Promontorio San Martín s/n, 39004 Santander, Spain E-mail: [email protected] (J.C.A); [email protected] (C.R.-C.); [email protected]; [email protected] (A.S). 4Instituto Español de Oceanografía, C.O. de Vigo, Subida Radio Faro 50. 36390 Vigo, Pontevedra, Spain. E-mail: [email protected] (C.-G.P.) Abstract A commented checklist containing 139 species of marine fishes recorded at the Galician Bank seamount is presented. The list is based on nine prospecting and research surveys carried out from 1980 to 2011 with different fishing gears. The ich- thyofauna list is diversified in 2 superclasses, 3 classes, 20 orders, 62 families and 113 genera. The largest family is Mac- rouridae, with 9 species, followed by Moridae, Stomiidae and Sternoptychidae with 7 species each.