Pediatric Psoriasis: from New Insights Into Pathogenesis to Updates on Treatment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Pdf/Bus Etat Des Lieux.Pdf Dermato-Allergol Bruxelles
1. w Volume 11, Number 2 April 2020 ISSN: 2081-9390 p. 113 - 223 DOI: 10.7241/ourd Issue online since Thursday April 02, 2020 Our Dermatology Online www.odermatol.com - Soraya Aouali, Imane Alouani, Hanane Ragragui, Nada Zizi, Siham Dikhaye A case of epithelioid angiosarcoma in a young man with chronic lymphedema - Iyda El Faqyr, Maria Dref, Sara Zahid, Jamila Oualla, Nabil Mansouri, Hanane Rais, Ouafa Hocar, Said Amal Syringocystadenoma papilliferum presented as an ulcerated nodule of the vulva in a patient with Neurofibromatosis type 1 - FMonisha Devi Selvakumari, Bittanakurike Narasappa Raghavendra, Anjan Kumar Patra A case of infantile Sweet’s syndrome - Anissa Zaouak, Leila Bouhajja, Houda Hammami, Samy Fenniche Penile annular lichen planus 2 / 2020 e-ISSN: 2081-9390 Editorial Pages DOI: 10.7241/ourd Quarterly published since 01/06/2010 years Our Dermatol Online www.odermatol.com Editor in Chief: Publisher: Piotr Brzeziński, MD Ph.D Our Dermatology Online Address: Address: ul. Braille’a 50B, 76200 Słupsk, Poland ul. Braille’a 50B, 76200 Słupsk, Poland tel. 48 692121516, fax. 48 598151829 tel. 48 692121516, fax. 48 598151829 e-mail: [email protected] e-mail: [email protected] Associate Editor: Ass. Prof. Viktoryia Kazlouskaya (USA) Indexed in: Universal Impact Factor for year 2012 is = 0.7319 system of opinion of scientific periodicals INDEX COPERNICUS (8,69) (Academic Search) EBSCO (Academic Search Premier) EBSCO MNiSW (kbn)-Ministerstwo Nauki i Szkolnictwa Wyższego (7.00) DOAJ (Directory of Open Acces Journals) Geneva Foundation -

Reseptregisteret 2013–2017 the Norwegian Prescription Database
LEGEMIDDELSTATISTIKK 2018:2 Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Christian Berg Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Utgitt av Folkehelseinstituttet/Published by Norwegian Institute of Public Health Område for Helsedata og digitalisering Avdeling for Legemiddelstatistikk Juni 2018 Tittel/Title: Legemiddelstatistikk 2018:2 Reseptregisteret 2013–2017 / The Norwegian Prescription Database 2013–2017 Forfattere/Authors: Christian Berg, redaktør/editor Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Acknowledgement: Julie D. W. Johansen (English text) Bestilling/Order: Rapporten kan lastes ned som pdf på Folkehelseinstituttets nettsider: www.fhi.no The report can be downloaded from www.fhi.no Grafisk design omslag: Fete Typer Ombrekking: Houston911 Kontaktinformasjon/Contact information: Folkehelseinstituttet/Norwegian Institute of Public Health Postboks 222 Skøyen N-0213 Oslo Tel: +47 21 07 70 00 ISSN: 1890-9647 ISBN: 978-82-8082-926-9 Sitering/Citation: Berg, C (red), Reseptregisteret 2013–2017 [The Norwegian Prescription Database 2013–2017] Legemiddelstatistikk 2018:2, Oslo, Norge: Folkehelseinstituttet, 2018. Tidligere utgaver / Previous editions: 2008: Reseptregisteret 2004–2007 / The Norwegian Prescription Database 2004–2007 2009: Legemiddelstatistikk 2009:2: Reseptregisteret 2004–2008 / The Norwegian Prescription Database 2004–2008 2010: Legemiddelstatistikk 2010:2: Reseptregisteret 2005–2009. Tema: Vanedannende legemidler / The Norwegian Prescription Database 2005–2009. -

Topically Used Herbal Products for the Treatment of Psoriasis – Mechanism of Action, Drug Delivery, Clinical Studies
Reviews 1447 Topically Used Herbal Products for the Treatment of Psoriasis – Mechanism of Action, Drug Delivery, Clinical Studies Authors Anna Herman1, Andrzej P. Herman 2 Affiliations 1 Faculty of Cosmetology, The Academy of Cosmetics and Health Care, Warsaw, Poland 2 Laboratory of Molecular Biology, The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Jabłonna, Poland Key words Abstract several electronic databases and literature refer- l" Psoriasis ! ences were used to summarize the current l" herbal products Psoriasis is a chronic inflammatory skin disease knowledge acquired on the basis of animal stud- l" keratinocyte hyper- characterized histologically by hyperproliferation ies and clinical trials regarding herbal products proliferation and aberrant differentiation of epidermal kerati- used to treat psoriasis topically. This review dis- l" inflammatory reaction l" skin barrier nocytes. A wide range of conventional medical cusses the mechanisms of herbal products activ- l" herbal drug delivery systems therapies to treat psoriasis is established, from ities through (1) inhibition of the keratinocyte hy- topical therapies and systemic medications perproliferation and inducing apoptosis, (2) inhi- through to phototherapy or combinations of bition of immune-inflammatory reaction, (3) those. However, most of these therapies have a suppression of phosphorylase kinase (PhK) activ- limited efficacy and may cause a number of side ity, and (4) inhibition of the hedgehog (Hh) sig- effects, including cutaneous atrophy, organ toxic- naling pathway. Moreover, the penetration of ity, carcinogenicity, and broadband immunosup- herbal products through the psoriatic skin barrier, pression, which are restricting their long-term novel herbal drug delivery systems in psoriasis use. Therefore, it would be desirable to use herbal treatment, and possible adverse effects of herbal products as an alternative treatment for psoriasis therapy are discussed. -

CMP 2 Psoriasis with Acitretin/Methotrexate
1CMP Psoriasis with Acitretin Name of Patient: Patient medication sensitivities/allergies: Patient identification e.g. ID number, date of birth: Current medication: Medical history: Independent Prescriber(s): Supplementary prescriber(s): Dr Sam Gibbs Jill Peters Dermatology Nurse Practitioner Contact details: 01473 704042 Contact details: 01473 704386 or 0787056578 [email protected] [email protected] Condition(s) to be treated: Aim of treatment: Psoriasis – scalp, flexural, guttate, small and large To achieve temporary clearance of the psoriasis or a maintainable plaque and palmer planter psoriasis level of clearance for the patient Medicines that my be prescribed by SP: Preparation Indication Dose schedule Specific indications for referral (All listed preparation may be back to the IP used as sole or conjunct therapy) Mild to potent topical Guttate or small plaque, As detailed in BNF Acute exacerbation or Failure to steroids palmo plantar psoriasis. Finger tip units respond to treatment Cautious use on face and flexural areas (moderate) Facial, scalp or flexural, palmo plantar psoriasis Coal Tar preparations Guttate or small plaque including scalp Dithranol preparations Moderate to large plaque As detailed in BNF as short stable psoriasis contact therapy Salicylic acid 2-10% in WSP Hyperkeratotic psoriasis on scalp or palmo plantar Vitamin D analogue Second line therapy to Use for 4 weeks and then change combined with potent topical moderate/large plaque to Vitamin D alone steroid psoriasis if unresponsive -
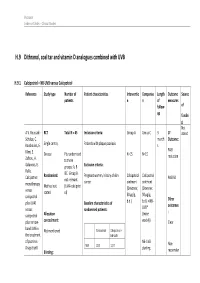
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each. -

Daivonex, Topical Ointment
NEW ZEALAND DATA SHEET 1 DAIVONEX® 50 microgram/gram topical ointment 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Daivonex® ointment contains calcipotriol 50 microgram per gram Daivonex® ointment contains the anhydrous form of calcipotriol. For full list of excipients, see section 6.1 List of excipients. 3 PHARMACEUTICAL FORM Daivonex® is a topical ointment. It is a smooth, white preservative free ointment base. 4 CLINICAL PARTICULARS 4.1 Indications Daivonex® ointment is indicated for the topical treatment of psoriasis vulgaris, including plaque psoriasis in adults and children (see section 4.4 Special warnings and precautions for use - paediatric population). In adult patients, Daivonex® ointment may also be used in combination with systemic acitretin or cyclosporin. 4.2 Dosage and method of administration Adults Daivonex® ointment therapy: Daivonex® ointment should be applied topically to the affected area once or twice daily (i.e. in the morning and/or in the evening). Initially, twice daily application of the ointment is usually preferred. Application may then be reduced to once daily, provided individual clinical response is satisfactory. After satisfactory improvement has occurred, treatment should be discontinued. If recurrence develops after reduction in frequency of application or after discontinuation, the treatment may be reinstituted at the initial dosage. Experience is lacking in the use of calcipotriol for periods longer than 1 year. The maximum recommended weekly dose of Daivonex® ointment is 100 g/week. When using a combination of ointment and cream the total maximum dose should not exceed 100 g per week. eDoc-000783454 - Version 1. 0 It should be noted that there are no long-term clinical studies assessing the safety of using Daivonex® ointment during exposure to sunlight. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Calcipotriol/Betamethasone Dipropionate: Daivobet®/Dovobet®
DRUG PROFILE Calcipotriol/betamethasone dipropionate: Daivobet®/Dovobet® Lyn C Guenther Psoriasis is a common skin condition that causes significant morbidity. Treatments can be 835 Richmond St., complex and time consuming. Calcipotriol plus betamethasone dipropionate ointment London, Ontario, (Daivobet® or Dovobet®) is a stable, convenient, once-daily topical treatment for psoriasis. N6A 3H7, Canada Tel.: +1 519 435 1738 Compared with its individual ingredients or tacalcitol, it has a faster speed of onset and Fax: +1 519 435 1740 greater efficacy. Consistent Psoriasis Area Severity Index reductions of approximately 40% [email protected] after 1 week and 70% after 4 weeks were seen in multicenter studies involving more than 6000 patients. After 4 weeks of once-daily therapy, approximately 50% of patients are clear or almost clear. This product is associated with a similar safety profile to betamethasone dipropionate ointment, 50% fewer cutaneous adverse events than calcipotriol ointment and 75% fewer than tacalcitol ointment. Psoriasis affects 0.3 to 2.6% of the population and receptor, acts as a heterodimer with the retinoid X has a significant impact on the patient’s quality of receptor (RXR) [9]. Keratinocytes and lympho- life [1–4]. A 1998 US survey of 17,488 National cytes have vitamin D receptors. In psoriasis, Psoriasis Foundation patient members showed that vitamin D analogues normalize differentiation psoriasis had a significant impact on psychosocial and proliferation, induce apoptosis in inflamma- activities (e.g., interacting in the workplace and tory cells, induce a T-helper (Th)1 to 2 switch with family/spouse, making and keeping friends, and are antiangiogenic [10–12]. -

Genital Psoriasis: a Systematic Literature Review on This Hidden Skin Disease
Acta Derm Venereol 2011 ; 91 ; 5-11 REVIEW ARTICLE Genital Psoriasis: A Systematic Literature Review on this Hidden Skin Disease Kim A. P. MEEUWIS'-^ Joanne A. DE HULLU^ Leon F. A. G. MASSUGER^ Peter C. M. VAN DE KERKHOF' and Michelle M. VAN ROSSUM' Departments of 'Dermatology and 'Obstetrics and Gynaecology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands It is well known that the genital skin may be affected by of inverse psoriasis (synonym: flexural or intertriginous psoriasis. However, little is known about the prevalence psoriasis) (2, 4, 5). and clinical appearance of genital psoriasis, and genital The external genital skin is generally classified as skin is often neglected in the treatment of psoriatic pa- flexural skin, although it forms a unique area compri- tients. We performed an extensive systematic literature sing different structures and types of epithelium. The search for evidence-based data on genital psoriasis with epithelium covering the different structures of the vulva respect to epidemiology, aetiology, clinical and histopat- changes from stratified, keratinised squamous cell epit- hological presentation, diagnosis and treatment. Three helium on the outer parts to mucosa on the innermost bibliographical databases (PubMed, EMBASE and the regions (5, 6). Similarly, the male genital epithelium Cochrane Library) were used as data sources. Fifty-nine has a different pattern of keratinisation throughout the articles on genital psoriasis were included. The results genital area. The prepuce forms the anatomical cover- show that psoriasis frequently affects the genital skin, ing of the glans penis and is the junction between the but that evidence-based data with respect to the efficacy mucosal surface of the glans and coronal sulcus and the and safety of treatments for genital psoriasis are extre- keratinised squamous cell epithelium of the remaining mely limited. -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

Papulosquamous Disorders (Psoriasis, Lichen Planus
PAPULOSQUAMOUS DISORDERS (PSORIASIS, LICHEN PLANUS & PITYRIASIS ROSEA) Objective of the lecture: • To know the definition of papulosquamous pattern. • To know the group of diseases known as papulosquamous diseases. • Psoriasis pathogenesis, clinical presentation, and management. • Lichen planus pathogenesis, clinical presentation, and management. • Pityriasis rosea pathogenesis, clinical presentation, and management. Done by team leader: Ghada Alhadlaq members: Rawan Alwadee & Deena AlNouwaiser. Revised by: Shrooq Alsomali Before you start.. CHECK THE EDITING FILE Sources: doctor’s slides and notes + Group B teamwork [ Color index: Important|doctor notes|Extra] Papulosquamous diseases: • The term squamous refers to scaling that represents thick Stratum Corneum and thus implies an abnormal keratinization process. - Keratinization is the differentiation of basal keratinocytes. - The basal keratinocytes as they move upward, they accumulate keratin inside them & their organelles totally die. • Papulosquamous diseases are typically characterized by scaly papules (papule = elevated lesion). • It could be papule or plaque, but the papulosquamous is the reaction pattern of the disease which means a reaction (inflammation) inside the skin (within the epidermis & dermis) to a specific thing that presents itself on the surface of the skin with a certain morphology. • Other disease patterns include psoriasiform, lichenoid, bullous, pustular as well as papulosquamous pattern & each has many differential diagnoses. :)الصدفية( Psoriasis • Chronic non-contagious polygenic multisystem inflammatory disease. • Psoriasis can be triggered by infections, trauma, stress and medications. • The characteristic lesion (classical psoriatic lesion) is a sharply demarcated erythematous scaly plaque that may be localized or generalized. • The natural history follows a chronic course with intermittent remissions. • Has two peaks in age of onset (bi-model age presentation): one at 20-30 years and a second at 40-50.