Host Range Testing Leptoypha Hospita: a Candidate Biological Control Agent for Privet (Ligustrum Spp.) in New Zealand
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forestiera Lace Bugs Leptoypha Elliptica (From Wheeler, 2002) (No Common Names) Leptoypha Elliptica Leptoypha Ilicis Contributor: Alfred G
Forestiera Lace Bugs Leptoypha elliptica (from Wheeler, 2002) (No common names) Leptoypha elliptica Leptoypha ilicis Contributor: Alfred G. Wheeler DESCRIPTION Taxonomy and Basic Description Both adults and nymphs of the family Tingidae are strictly phytophagous. Lace bugs are mostly host restricted, developing on plants of only one genus or several related genera. They are found mainly on woody plants, the nymphs feeding on lower surfaces of leaves. Their feeding on mesophyll tissues usually results in a bleached or chlorotic appearance of the upper leaf surface and dark spots of excrement on the lower surface. Some lace bugs feed on herbaceous vascular plants and species of one genus (Acalypta) feed mainly on mosses. Lace bugs associated with small trees and shrubs of the genus Forestiera (olive family; Oleaceae) include two morphologically similar species of Leptoypha. McAtee described L. elliptica from Texas in 1917; L. ilicis was described in 1919 from Georgia by Drake. Only recently was the specific name of the latter species shown to be a Leptoypha ilicis (from Wheeler, 2002) misnomer. This lace bug was named ilicis because of its assumed host association: Ilex sp. Its actual hosts are species of Forestiera, the plant mentioned in the original description having been misidentified. Forestiera species bear a superficial resemblance to certain species of Ilex (holly; family Aquifoliaceae). Leptoypha species are small (about 3 mm long or 0.12 inches), elongate or narrowly oblong, and straw yellow to reddish brown. A hood (prothoracic covering of the head) and paranota (lateral expansions of pronotum), which characterize many lace bugs, are lacking. Also absent is the translucent lacy appearance of the forewings (hemelytra) that is typical of the Tingidae; elevations of the forewings also are absent. -

'Ivory Silk' Japanese Tree Lilac
Fact Sheet ST-611 October 1994 Syringa reticulata ‘Ivory Silk’ ‘Ivory Silk’ Japanese Tree Lilac1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Although a Lilac, this member of the species is quite different in appearance than those with which gardeners are more familiar (Fig. 1). Its upright habit varies from symmetrical to irregular but is more consistent than the species. Cultivars including ‘Ivory Silk’ and ‘Summer Snow’ could be used instead of the species due to the more consistent habit and more flowers. ‘Ivory Silk’ grows well only in USDA hardiness zones 3 through six (perhaps into 7) and has an oval or pyramidal form when young but spreads to a rounded shape as it grows older. This is a very large shrub or small tree, reaching a height of about 20 to 30 feet with a 15-foot-spread. The huge clusters of creamy white flowers, borne in early summer for about two weeks, are the main ornamental feature but lack the fragrance of the spring-blooming Lilacs -- this Lilac’s fragrance is more suggestive of Privet. GENERAL INFORMATION Scientific name: Syringa reticulata ‘Ivory Silk’ Pronunciation: sih-RING-guh reh-tick-yoo-LAY-tuh Common name(s): ‘Ivory Silk’ Japanese Tree Lilac Family: Oleaceae Figure 1. Mature ‘Ivory Silk’ Japanese Tree Lilac. USDA hardiness zones: 3A through 7A (Fig. 2) Origin: not native to North America sidewalk cutout (tree pit); residential street tree; tree Uses: container or above-ground planter; large has been successfully grown in urban areas where air parking lot islands (> 200 square feet in size); wide pollution, poor drainage, compacted soil, and/or tree lawns (>6 feet wide); medium-sized tree lawns drought are common (4-6 feet wide); recommended for buffer strips around Availability: somewhat available, may have to go out parking lots or for median strip plantings in the of the region to find the tree highway; near a deck or patio; screen; trainable as a standard; narrow tree lawns (3-4 feet wide); specimen; 1. -

Priogymnanthus Colombianus (Oleaceae), a New Species and First Record of Genus to Colombia
Phytotaxa 399 (3): 195–202 ISSN 1179-3155 (print edition) https://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2019 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.399.3.3 Priogymnanthus colombianus (Oleaceae), a new species and first record of genus to Colombia JOSÉ LUIS FERNÁNDEZ-ALONSO1* & PAULA ANDREA MORALES MORALES2 1Real Jardín Botánico –CSIC. Departamento de Biodiversidad y Conservación. Plaza de Murillo 2, 28014 Madrid. España. Email: [email protected]. ORCID ID: http://orcid.org/0000-0002-1701-480X 2Herbario Universidad de Antioquia, Facultad de Ciencias Exactas y Naturales, Apartado aéreo 1226, Medellín. Colombia. Email: [email protected]. ORCID ID: http://orcid.org/0000-0002-9167-6027 *Corresponding author Abstract Priogymnanthus colombianus, a new species and the first record of the South American genus of Oleaceae for Colombia is described and illustrated also we present a dichotomic key for the known species of genus. The new species differs from the three knowns for Priogymnanthus by: leaves oblong or oblong-elliptic, completely glabrous, petioles 10–17 (19) mm; inflorescences 15–20 (25) mm in length, with glabrous rachis, anthers about 3 mm length; fruits (10) 12–15 mm in diameter. P. colombianus occurs on premontane and dry forest in Colombia between 719 and 1213 m of elevation. Based on general threats to its ecosystems and few records found, we categorize the species as EN (endangered) following IUCN criteria. Resumen Se describe e ilustra Priogymnanthus colombianus, una nueva especie y primer representante de este género suramericano de Oleaceae en Colombia, y se presenta una clave dicotómica para la identificación de las especies conocidas del género. -

Proceedings of the United States National Museum
Proceedings of the United States National Museum SMITHSONIAN INSTITUTION • WASHINGTON, D.C. Volume 112 I960 Number 3431 LACE-BUG GENERA OF THE WORLD (HEMIPTERA: TINGIDAE) « By Carl J. Drake and Florence A. Ruhoff Introduction A treatise of the generic names of the family Tingidae from a global standpoint embodies problems similar to those frequently encountered in corresponding studies in other animal groups. The more im- portant criteria, including such basic desiderata as fixation of type species, synonyms, priority, and dates of technical publications implicate questions concomitant with recent trends toward the clarification and stabilization of zoological nomenclature. Zoogeography, predicated and authenticated on the generic level by the distribution of genera and species, is portrayed here by means of tables, charts, and maps of the tingifauna of the world. This visual pattern of distribution helps one to form a more vivid concept of the family and its hierarchic levels of subfamilies and genera. To a limited extent the data indicate distributional concentrations and probable centers of evolution and dispersal paths of genera. The phylogenetic relationship of genera is not discussed. The present treatise recognizes 216 genera (plus 79 synonyms, homonyms, and emendations) of the Tingidae of the world and gives 1 Research for this paper was supported In part by the National Science Foundation, grant No. 4095. 2 PROCEEDINGS OF THE NATIONAL MUSEUM vol. 112 the figure of 1,767 as the approximate number of species now recog- nized. These figures, collated with similar categories in Lethierry and Severin (1896), show that there has been an increase of many genera and hundreds of species of Tingidae during the past three- quarters of a century. -

THE JEPSON GLOBE a Newsletter from the Friends of the Jepson Herbarium
THE JEPSON GLOBE A Newsletter from the Friends of The Jepson Herbarium VOLUME 26 NUMBER 1, Spring 2016 Curator’s Column: Museomics The Jepson Manual: Vascular Reveals Secrets of the Dead Plants of California, Second By Bruce G. Baldwin Edition: Supplement III Over the last decade, herbaria By Bruce G. Baldwin have received well-deserved public- The latest set of revisions to The Jep- ity as treasure troves of undiscovered son Manual, second edition (TJM2) and biodiversity, with the recognition that the Jepson eFlora was released online most “new” species named in the last in December 2015. The rapid pace of half-century have long resided in col- discovery and description of vascular lections prior to their detection and plant taxa that are new-to-science for original description. The prospect also California and the rarity and endanger- has emerged for unlocking the secrets of ment of most of those new taxa have plants and other organisms that no lon- warranted prioritization of revisions ger share our planet as living organisms that incorporate such diversity — and and, sadly, reside only in collections. Map of California, split apart to show newly introduced, putatively aggressive Technological advances that now al- the Regions of the Jepson eFlora. invasives — so that detection of such low for DNA sequencing on a genomic Source: Jepson Flora Project. plants in the field and in collections scale also are well suited for studying Regional dichotomous keys now is not impeded. The continuing taxo- old, highly degraded specimens, as re- nomic reorganization of genera and, to cent reconstruction of the Neanderthal available for the Jepson eFlora some extent, families in order to reflect genome has shown. -

POTENTIAL of a NATIVE LACE BUG (LEPTOYPHA MUTICA) for BIOLOGICAL CONTROL of CHINESE PRIVET (LIGUSTRUM SINENSE) by JESSICA A
POTENTIAL OF A NATIVE LACE BUG (LEPTOYPHA MUTICA) FOR BIOLOGICAL CONTROL OF CHINESE PRIVET (LIGUSTRUM SINENSE) By JESSICA A. KALINA (Under the Direction of S. Kristine Braman and James L. Hanula) Abstract This work was done to better understand the lace bug Leptoypha mutica Say (Hemiptera: Tingidae) and its potential for biological control of Chinese privet, Ligustrum sinense Lour (Oleaceae). L. mutica host utilization of Chinese privet was confirmed in a no choice test. In choice tests with plant species Chinese privet, swamp privet (Foresteria acuminata Michx), and green ash (Fraxinus pennsylvanica Marsh) L. mutica preferred green ash. The duration of development of L. mutica on Chinese privet from egg to adult ranged from 24.4 to 57.1 days at o o o o the temperatures: 20 C, 24 C, 28 C, and 32 C. Estimated threshold temperatures (To) and calculated thermal unit requirements (K) for development of egg, nymphal, and complete development were 11.0oC, 9.9oC, 10.5oC and 211.9, 326.8, 527.4 degree-days (DD). The only suspected specific Leptoypha spp. natural enemy found was a mymarid wasp, but was not concluded to be limiting factor for L. hospita’s success. INDEX WORDS: Chinese privet, Ligustrum sinense, Leptoypha mutica, biological control, invasive, biology, host study, no choice study, choice study, natural enemy complex. _________________________________________________________ POTENTIAL OF NATIVE LACE BUG (LEPTOYPHA MUTICA) FOR BIOLOGICAL CONTROL OF CHINESE PRIVET (LIGUSTRUM SINENSE) By JESSICA A. KALINA B.S., The University of Georgia, 2012 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE GRIFFIN, GEORGIA 2013 ©2013 Jessica A. -
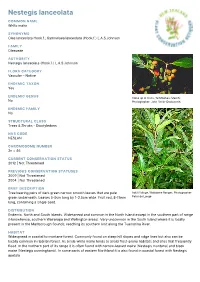
Nestegis Lanceolata
Nestegis lanceolata COMMON NAME White maire SYNONYMS Olea lanceolata Hook.f.; Gymnelaea lanceolata (Hook.f.) L.A.S.Johnson FAMILY Oleaceae AUTHORITY Nestegis lanceolata (Hook.f.) L.A.S.Johnson FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Close up of fruits, Te Moehau (March). No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Dicotyledons NVS CODE NESLAN CHROMOSOME NUMBER 2n = 46 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened BRIEF DESCRIPTION Tree bearing pairs of dark green narrow smooth leaves that are pale Adult foliage, Waitakere Ranges. Photographer: green underneath. Leaves 5-9cm long by 1-2.5cm wide. Fruit red, 8-11mm Peter de Lange long, containing a single seed. DISTRIBUTION Endemic. North and South Islands. Widespread and common in the North Island except in the southern part of range (Horowhenua, southern Wairarapa and Wellington areas). Very uncommon in the South Island where it is locally present in the Marlborough Sounds, reaching its southern limit along the Tuamarina River. HABITAT Widespread in coastal to montane forest. Commonly found on steep hill slopes and ridge lines but also can be locally common in riparian forest. As a rule white maire tends to avoid frost-prone habitats and sites that frequently flood. In the northern part of its range it is often found with narrow-leaved maire (Nestegis montana) and black maire (Nestegis cunninghamii). In some parts of eastern Northland it is also found in coastal forest with Nestegis apetala. FEATURES Stout gynodioecious spreading tree up to 20 m tall usually forming a domed canopy; trunk up to c. -

Developmental Biology of Leptoypha Mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae) Author(S): J
Developmental Biology of Leptoypha mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae) Author(s): J. Kalina, S.K. Braman, and J.L. Hanula Source: Journal of Entomological Science, 52(2):154-160. Published By: Georgia Entomological Society https://doi.org/10.18474/JES16-28.1 URL: http://www.bioone.org/doi/full/10.18474/JES16-28.1 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Developmental Biology of Leptoypha mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae)1 J. Kalina2, S.K. Braman3, and J.L. Hanula4 University of Georgia, Department of Entomology, 413 Biological Sciences Building, Athens, Georgia 30602 USA J. Entomol. Sci. 52(2): 154–160 (April 2017) Abstract The native lace bug, Leptoypha mutica Say (Hemiptera: Tingidae), has demonstrated potential as an insect biological control agent of invasive Chinese privet (Ligustrum sinense Lour). -

Torus-Bearing Pit Membranes in Species of Osmanthus
IAWA Journal, Vol. 31 (2), 2010: 217–226 TORUS-BEARING PIT MEMBRANES IN SPECIES OF OSMANTHUS Roland Dute1, 5, David Rabaey2, John Allison1 and Steven Jansen3, 4 SUMMARY Torus thickenings of pit membranes are found not only in gymnosperms, but also in certain genera of dicotyledons. One such genus is Osmanthus. Wood from 17 species of Osmanthus was searched for tori. Fourteen spe- cies from three of the four sections investigated possessed these thick- enings. Ten of the species represent new records. Only the three New Caledonian species of Section Notosmanthus lacked tori. This observation in combination with other factors serves to isolate this section from the remainder of the genus. Key words: Notelaea, Osmanthus, pit membrane, torus. INTRODUCTION Osmanthus is a member of the Oleaceae (Olive Family), a moderate-sized family with circa 24 genera and 615 spp. (Stevens 2001). There are 33 natural species within the genus (Table 1; Xiang et al. 2008), and they show a tropical to temperate distribution (Bailey & Bailey 1976; Denk et al. 2001; Xiang et al. 2008). Generally, individual shrubs are found in moist forests (Hsieh et al. 1998; Chou et al. 2000; Denk et al. 2001), frequently on steep slopes (Green 1958). In some instances populations are sub- ject to monsoon conditions and hence to periods of relatively low rainfall (Yang et al. 2008; Hua 2008). Tori are thickenings of intervascular pit membranes found not only in gymnosperm, but also in angiosperm wood, including that of Osmanthus (Dute et al. 2008; Dute et al. 2010). Tori were first observed in O. fragrans, O. -

The Vegetation of the Western Blue Mountains Including the Capertee, Coxs, Jenolan & Gurnang Areas
Department of Environment and Conservation (NSW) The Vegetation of the Western Blue Mountains including the Capertee, Coxs, Jenolan & Gurnang Areas Volume 1: Technical Report Hawkesbury-Nepean CMA CATCHMENT MANAGEMENT AUTHORITY The Vegetation of the Western Blue Mountains (including the Capertee, Cox’s, Jenolan and Gurnang Areas) Volume 1: Technical Report (Final V1.1) Project funded by the Hawkesbury – Nepean Catchment Management Authority Information and Assessment Section Metropolitan Branch Environmental Protection and Regulation Division Department of Environment and Conservation July 2006 ACKNOWLEDGMENTS This project has been completed by the Special thanks to: Information and Assessment Section, Metropolitan Branch. The numerous land owners including State Forests of NSW who allowed access to their Section Head, Information and Assessment properties. Julie Ravallion The Department of Natural Resources, Forests NSW and Hawkesbury – Nepean CMA for Coordinator, Bioregional Data Group comments on early drafts. Daniel Connolly This report should be referenced as follows: Vegetation Project Officer DEC (2006) The Vegetation of the Western Blue Mountains. Unpublished report funded by Greg Steenbeeke the Hawkesbury – Nepean Catchment Management Authority. Department of GIS, Data Management and Database Environment and Conservation, Hurstville. Coordination Peter Ewin Photos Kylie Madden Vegetation community profile photographs by Greg Steenbeeke Greg Steenbeeke unless otherwise noted. Feature cover photo by Greg Steenbeeke. All Logistics -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear. -

Structural Diversity and Contrasted Evolution of Cytoplasmic Genomes in Flowering Plants :A Phylogenomic Approach in Oleaceae Celine Van De Paer
Structural diversity and contrasted evolution of cytoplasmic genomes in flowering plants :a phylogenomic approach in Oleaceae Celine van de Paer To cite this version: Celine van de Paer. Structural diversity and contrasted evolution of cytoplasmic genomes in flowering plants : a phylogenomic approach in Oleaceae. Vegetal Biology. Université Paul Sabatier - Toulouse III, 2017. English. NNT : 2017TOU30228. tel-02325872 HAL Id: tel-02325872 https://tel.archives-ouvertes.fr/tel-02325872 Submitted on 22 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REMERCIEMENTS Remerciements Mes premiers remerciements s'adressent à mon directeur de thèse GUILLAUME BESNARD. Tout d'abord, merci Guillaume de m'avoir proposé ce sujet de thèse sur la famille des Oleaceae. Merci pour ton enthousiasme et ta passion pour la recherche qui m'ont véritablement portée pendant ces trois années. C'était un vrai plaisir de travailler à tes côtés. Moi qui étais focalisée sur les systèmes de reproduction chez les plantes, tu m'as ouvert à un nouveau domaine de la recherche tout aussi intéressant qui est l'évolution moléculaire (même si je suis loin de maîtriser tous les concepts...). Tu as toujours été bienveillant et à l'écoute, je t'en remercie.