Chapter 1: Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gammaherpesvirus in Semi-Domesticated Reindeer (Rangifer Tarandus Tarandus) in Finnmark County, Norway
FACULTY OF BIOSCIENCES, FISHERIES AND ECONOMICS DEPARTMENT OF ARCTIC AND MARINE BIOLOGY Gammaherpesvirus in semi-domesticated reindeer (Rangifer tarandus tarandus) in Finnmark County, Norway Hanne Marie Ihlebæk BIO-3900 Master`s thesis in Arctic natural resource management and agriculture May 2010 2 Gammaherpesvirus in semi-domesticated reindeer (Rangifer tarandus tarandus) in Finnmark County, Norway Hanne Marie Ihlebæk May 2010 In collaboration with The Norwegian School of Veterinary Science Section of Arctic Veterinary Medicine 3 4 Acknowledgments This thesis was part of the research project Reinhelse 2008–2010, under direction of The Norwegian School of Veterinary Science, Section of Arctic Veterinary Medicine (SAV), Tromsø. The project was partly funded by the Norwegian Reindeer Development Fund (RUF). Several people have contributed with help on this thesis. First of all I owe a very special thank to my main supervisor Morten Tryland for giving me the opportunity to work with this project and for all guidance and trust throughout this period. I also wish to thank my supervisor Willy Hemmingsen for all helpful advises and comments on the writing process of my thesis. My special acknowledgement also goes to the laboratory personal and especially Eva Marie Breines for invaluable guidance in the laboratory, to Carlos das Neves for excellent help with the PCR results and to Eystein Skjerve for help with the statistical model, Kjetil Åsbakk for guiding me into JMP and Trond Elde for genuine help with an easy going database. Thanks to Maret Haetta, Carlos and Alina Evans for enjoyable company at fieldwork. At last I will thank my parents for all support and love. -

Animals of Africa
Silver 49 Bronze 26 Gold 59 Copper 17 Animals of Africa _______________________________________________Diamond 80 PYGMY ANTELOPES Klipspringer Common oribi Haggard oribi Gold 59 Bronze 26 Silver 49 Copper 17 Bronze 26 Silver 49 Gold 61 Copper 17 Diamond 80 Diamond 80 Steenbok 1 234 5 _______________________________________________ _______________________________________________ Cape grysbok BIG CATS LECHWE, KOB, PUKU Sharpe grysbok African lion 1 2 2 2 Common lechwe Livingstone suni African leopard***** Kafue Flats lechwe East African suni African cheetah***** _______________________________________________ Red lechwe Royal antelope SMALL CATS & AFRICAN CIVET Black lechwe Bates pygmy antelope Serval Nile lechwe 1 1 2 2 4 _______________________________________________ Caracal 2 White-eared kob DIK-DIKS African wild cat Uganda kob Salt dik-dik African golden cat CentralAfrican kob Harar dik-dik 1 2 2 African civet _______________________________________________ Western kob (Buffon) Guenther dik-dik HYENAS Puku Kirk dik-dik Spotted hyena 1 1 1 _______________________________________________ Damara dik-dik REEDBUCKS & RHEBOK Brown hyena Phillips dik-dik Common reedbuck _______________________________________________ _______________________________________________African striped hyena Eastern bohor reedbuck BUSH DUIKERS THICK-SKINNED GAME Abyssinian bohor reedbuck Southern bush duiker _______________________________________________African elephant 1 1 1 Sudan bohor reedbuck Angolan bush duiker (closed) 1 122 2 Black rhinoceros** *** Nigerian -

Cervid Mixed-Species Table That Was Included in the 2014 Cervid RC
Appendix III. Cervid Mixed Species Attempts (Successful) Species Birds Ungulates Small Mammals Alces alces Trumpeter Swans Moose Axis axis Saurus Crane, Stanley Crane, Turkey, Sandhill Crane Sambar, Nilgai, Mouflon, Indian Rhino, Przewalski Horse, Sable, Gemsbok, Addax, Fallow Deer, Waterbuck, Persian Spotted Deer Goitered Gazelle, Reeves Muntjac, Blackbuck, Whitetailed deer Axis calamianensis Pronghorn, Bighorned Sheep Calamian Deer Axis kuhili Kuhl’s or Bawean Deer Axis porcinus Saurus Crane Sika, Sambar, Pere David's Deer, Wisent, Waterbuffalo, Muntjac Hog Deer Capreolus capreolus Western Roe Deer Cervus albirostris Urial, Markhor, Fallow Deer, MacNeil's Deer, Barbary Deer, Bactrian Wapiti, Wisent, Banteng, Sambar, Pere White-lipped Deer David's Deer, Sika Cervus alfredi Philipine Spotted Deer Cervus duvauceli Saurus Crane Mouflon, Goitered Gazelle, Axis Deer, Indian Rhino, Indian Muntjac, Sika, Nilgai, Sambar Barasingha Cervus elaphus Turkey, Roadrunner Sand Gazelle, Fallow Deer, White-lipped Deer, Axis Deer, Sika, Scimitar-horned Oryx, Addra Gazelle, Ankole, Red Deer or Elk Dromedary Camel, Bison, Pronghorn, Giraffe, Grant's Zebra, Wildebeest, Addax, Blesbok, Bontebok Cervus eldii Urial, Markhor, Sambar, Sika, Wisent, Waterbuffalo Burmese Brow-antlered Deer Cervus nippon Saurus Crane, Pheasant Mouflon, Urial, Markhor, Hog Deer, Sambar, Barasingha, Nilgai, Wisent, Pere David's Deer Sika 52 Cervus unicolor Mouflon, Urial, Markhor, Barasingha, Nilgai, Rusa, Sika, Indian Rhino Sambar Dama dama Rhea Llama, Tapirs European Fallow Deer -

CAMEROON Savanna & Forest
Serving Sportsmen Since 1952 CAMEROON Savanna & Forest Hunting safaris in Cameroon are conducted in both the Savannah region which is generally in the North of the country and the Forest in the Southern regions. The areas are safe and easily accessible. Cameroon is an incredible country for big game hunting and we arrange safaris in the best hunting areas of the country. Safari Outfitters has represented this superb hunting outfitter Corporate Headquarters longer then any of our other African outfitters with 143 South Bent Street, Suite D the exception of one. So you will see that we know Powell, WY 82435 them very well and are 100% confident in their 307-587-5596 areas, success, PH’s and camps. www.safari1.com cf Breaking Records... it’s what we do! Savannah Forest Without a doubt, the magnificent Lord Derby or Arguably the most strikingly beautiful antelope in Giant Eland is one the top trophies in all of Africa all of Africa, the Bongo is high on the list of those and the largest of the antelope group. This guy can who yearn to hunt wild places. Local Pygmies trot all day and jump a 6 foot fence from a standing trackers and their small dogs are used to hunt start. In addition to Lord Derby Eland, game such Bongo in the dense rainforest and they do an as Western Roan, West African Savannah Buffalo, amazing job. The dogs are used to hold the animal Korrigum, Central Kob, Sing-sing Waterbuck and in place while the hunter takes the shot. Without others may hunted in the Niwa and Vina hunting dogs, the Bongo would just keep running and areas. -

Transboundary Species Project
TRANSBOUNDARY SPECIES PROJECT ROAN, SABLE AND TSESSEBE Rowan B. Martin Species Report for Roan, Sable and Tsessebe in support of The Transboundary Mammal Project of the Ministry of Environment and Tourism, Namibia facilitated by The Namibia Nature Foundation and World Wildlife Fund Living in a Finite Environment (LIFE) Programme Cover picture adapted from the illustrations by Clare Abbott in The Mammals of the Southern African Subregion by Reay H.N. Smithers Published by the University of Pretoria Republic of South Africa 1983 Transboundary Species Project – Background Study Roan, Sable and Tsessebe CONTENTS 1. BIOLOGICAL INFORMATION ...................................... 1 a. Taxonomy ..................................................... 1 b. Physical description .............................................. 3 c. Habitat ....................................................... 6 d. Reproduction and Population Dynamics ............................. 12 e. Distribution ................................................... 14 f. Numbers ..................................................... 24 g. Behaviour .................................................... 38 h. Limiting Factors ............................................... 40 2. SIGNIFICANCE OF THE THREE SPECIES ........................... 43 a. Conservation Significance ........................................ 43 b. Economic Significance ........................................... 44 3. STAKEHOLDING ................................................. 48 a. Stakeholders ................................................. -

U.S. Fish and Wildlife Service Affirms Protection for Three African Antelope Species Under the ESA
May 31, 2013 Contact: Claire Cassel 703-358-2357 [email protected] U.S. Fish and Wildlife Service Affirms Protection for Three African Antelope Species under the ESA U.S. captive-bred specimens of three endangered African antelope species — the scimitar-horned oryx, dama gazelle and addax – continue to warrant protection under the Endangered Species Act (ESA), the U.S. Fish and Wildlife Service announced today. In findings made in response to two petitions seeking the removal of U.S. captive populations of these endangered species from the Federal List of Endangered and Threatened Wildlife, the Service has determined that providing separate legal status to captive specimens of protected species is not permissible under the ESA. The findings will not impact the current, legal operation of game ranching operations and are consistent with the Service’s longstanding practice for applying the protections of the ESA to captive individuals of species that are threatened or endangered in the wild. The findings were submitted to the Federal Register on May 31. In 2005, the Service added these three antelope species to the Federal List of Endangered and Threatened Wildlife. The species all inhabit the sparse desert regions of northern Africa. Populations for all three species have been greatly reduced as a result of habitat loss, military activity and uncontrolled killing. In the wild, the scimitar-horned oryx may be extirpated, and the other two species are each estimated to number fewer than 500 individuals. Each of the three species occurs in greater numbers in captivity than in the wild, and many of the captive animals are held on private ranches in the United States. -

Addax (Addax Nasomaculatus), Scimitar- Horned Oryx (Oryx Dammah), Arabian Oryx (Oryx Leucoryx), and Sable Antelope (Hippotragus Niger)
Zoo Biology 20:47–54 (2001) Comparisons Among Selected Neonatal Biomedical Parameters of Four Species of Semi-Free Ranging Hippotragini: Addax (Addax nasomaculatus), Scimitar- horned Oryx (Oryx dammah), Arabian Oryx (Oryx leucoryx), and Sable Antelope (Hippotragus niger) Shannon T. Ferrell,1* Robin W. Radcliffe,1 Rodney Marsh,1 Cathy B. Thurman,1 Christine M. Cartwright,1 Thomas W.J. De Maar,2 Evan S Blumer,3 Ed Spevak,4 and Steven A. Osofsky5 1Fossil Rim Wildlife Center, Department of Animal Health Services, Glen Rose, Texas 2Ol Jogi, Ltd., Nanyuki, Kenya 3The Wilds, Cumberland, Ohio 4Wildlife Conservation Society, Bronx, New York 5World Wildlife Fund, Washington, D.C. Basic biomedical data from 164 neonates of four species of the tribe Hippotragini, addax (Addax nasomaculatus), scimitar-horned oryx (Oryx dammah), Arabian oryx (Oryx leucoryx), and sable antelope (Hippotragus niger), were compared at one zoological institution over a 9-year period. Measured biomedical parameters included body weight, temperature, pulse and respiratory rates, packed cell vol- ume (PCV), total plasma protein, glucose, IgG assessment via zinc sulfate tur- bidity, and white blood cell count with differential. All species were maintained in a semi-free ranging setting with the same diet, available shelter, and opportu- nity for social interaction. Based on clinical and field observations, all neonates used in the study were believed to be at least 24 hr old, to have bonded with the dam, and to have no obvious physical abnormalities. Median body weights were similar only in the addax and Arabian oryx with sable antelope having the larg- est median body weight. No significant differences in rectal temperatures or pulse rates were found among species. -

Name Address • Phone Number • Email Address EDUCATION
Name Address • Phone number • Email address EDUCATION_______________________________________________________________________________ • B.S. in Biology with a specialization in Zoology –University (graduated May 2013) • Associate’s of Science – College (graduated May 2010) • Equine Health – College (August 2007 – June 2008) • Culinary Arts Certificate – Career Center (graduated May 2007) • Leadership, Process Improvement, and Team Building Certificate – AAZK (2018) EXPERIENCE_______________________________________________________________________________ Wild Animal Keeper/Temporary Wild Animal Keeper/Replacement Keeper: December 2014 – December 2015; July 2017-December 2017; December 2018-Current Zoo, Place • Responsible for daily husbandry care (diet prep, feeding, cleaning, record-keeping, enrichment, and administering medication) for lemurs, alpaca, capybara, Andean condor, Himalayan tahr, Siberian musk deer, axolotl, cave snakes, seba’s short-tailed bats, straw colored fruit bats, pygmy slow loris, leaf-tailed geckos, spiny stick insects, leafcutter ants, tawny frog mouths, various waterfowl, silver fulu, wood turtle, box turtle, galapagos tortoise, golden lion tamarins, green tree pythons, freshwater stingrays, partula snails, chameleons, naked mole rats, red- eyed tree frogs, mata-mata turtle, and native ohio songbirds. • Operant conditioning for a variety of species • Trained on water quality testing for freshwater and salt water systems Horticulturist/Seasonal Horticulturalist: April 2017-July 2017; December 2017-December 2018 -

Mixed-Species Exhibits with Pigs (Suidae)
Mixed-species exhibits with Pigs (Suidae) Written by KRISZTIÁN SVÁBIK Team Leader, Toni’s Zoo, Rothenburg, Luzern, Switzerland Email: [email protected] 9th May 2021 Cover photo © Krisztián Svábik Mixed-species exhibits with Pigs (Suidae) 1 CONTENTS INTRODUCTION ........................................................................................................... 3 Use of space and enclosure furnishings ................................................................... 3 Feeding ..................................................................................................................... 3 Breeding ................................................................................................................... 4 Choice of species and individuals ............................................................................ 4 List of mixed-species exhibits involving Suids ........................................................ 5 LIST OF SPECIES COMBINATIONS – SUIDAE .......................................................... 6 Sulawesi Babirusa, Babyrousa celebensis ...............................................................7 Common Warthog, Phacochoerus africanus ......................................................... 8 Giant Forest Hog, Hylochoerus meinertzhageni ..................................................10 Bushpig, Potamochoerus larvatus ........................................................................ 11 Red River Hog, Potamochoerus porcus ............................................................... -

List of 28 Orders, 129 Families, 598 Genera and 1121 Species in Mammal Images Library 31 December 2013
What the American Society of Mammalogists has in the images library LIST OF 28 ORDERS, 129 FAMILIES, 598 GENERA AND 1121 SPECIES IN MAMMAL IMAGES LIBRARY 31 DECEMBER 2013 AFROSORICIDA (5 genera, 5 species) – golden moles and tenrecs CHRYSOCHLORIDAE - golden moles Chrysospalax villosus - Rough-haired Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus – Lowland Streaked Tenrec 3. Microgale dobsoni - Dobson’s Shrew Tenrec 4. Tenrec ecaudatus – Tailless Tenrec ARTIODACTYLA (83 genera, 142 species) – paraxonic (mostly even-toed) ungulates ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BOVIDAE (46 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Impala 3. Alcelaphus buselaphus - Hartebeest 4. Alcelaphus caama – Red Hartebeest 5. Ammotragus lervia - Barbary Sheep 6. Antidorcas marsupialis - Springbok 7. Antilope cervicapra – Blackbuck 8. Beatragus hunter – Hunter’s Hartebeest 9. Bison bison - American Bison 10. Bison bonasus - European Bison 11. Bos frontalis - Gaur 12. Bos javanicus - Banteng 13. Bos taurus -Auroch 14. Boselaphus tragocamelus - Nilgai 15. Bubalus bubalis - Water Buffalo 16. Bubalus depressicornis - Anoa 17. Bubalus quarlesi - Mountain Anoa 18. Budorcas taxicolor - Takin 19. Capra caucasica - Tur 20. Capra falconeri - Markhor 21. Capra hircus - Goat 22. Capra nubiana – Nubian Ibex 23. Capra pyrenaica – Spanish Ibex 24. Capricornis crispus – Japanese Serow 25. Cephalophus jentinki - Jentink's Duiker 26. Cephalophus natalensis – Red Duiker 1 What the American Society of Mammalogists has in the images library 27. Cephalophus niger – Black Duiker 28. Cephalophus rufilatus – Red-flanked Duiker 29. Cephalophus silvicultor - Yellow-backed Duiker 30. Cephalophus zebra - Zebra Duiker 31. Connochaetes gnou - Black Wildebeest 32. Connochaetes taurinus - Blue Wildebeest 33. Damaliscus korrigum – Topi 34. -
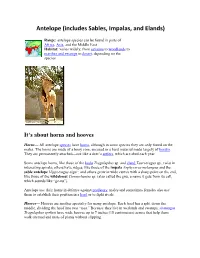
Antelope (Includes Sables, Impalas, and Elands)
Antelope (includes Sables, Impalas, and Elands) Range: antelope species can be found in parts of Africa, Asia, and the Middle East Habitat: varies widely, from savanna to woodlands to marshes and swamps to desert, depending on the species It’s about horns and hooves Horns— All antelope species have horns, although in some species they are only found on the males. The horns are made of a bony core, encased in a hard material made largely of keratin. They are permanently attached—not like a deer’s antlers, which are shed each year. Some antelope horns, like those of the kudu Tragelaphus sp. and eland Taurotragus sp., twist in interesting spirals; others have ridges, like those of the impala Aephyceros melampus and the sable antelope Hippotragus niger; and others grow in wide curves with a sharp point on the end, like those of the wildebeest Connochaetes sp. (also called the gnu, a name it gets from its call, which sounds like “ge-nu”). Antelope use their horns in defense against predators; males and sometimes females also use them to establish their position in a herd or to fight rivals Hooves— Hooves are another specialty for many antelope. Each hoof has a split down the middle, dividing the hoof into two “toes.” Because they live in wetlands and swamps, sitatungas Tragelaphus spekeii have wide hooves up to 7 inches (18 centimeters) across that help them walk on mud and mats of plants without slipping. Nile lechwes Kobus magaceros, which also live in swampy areas, have long, pointed hooves to give them sure footing in the water. -

GNUSLETTER Vol 37#2.Pdf
GNUSLETTER Volume 37 / Number 2 December 2020 ANTELOPE SPECIALIST GROUP IUCN Species Survival Commission Antelope Specialist Group GNUSLETTER is the biannual newsletter of the IUCN Species Survival Commission Antelope Specialist Group (ASG). First published in 1982 by first ASG Chair Richard D. Estes, the intent of GNUSLETTER, then and today, is the dissemination of reports and information regarding antelopes and their conservation. ASG Members are an important network of individuals and experts working across disciplines throughout Africa, Asia and America. Contributions (original articles, field notes, other material relevant to antelope biology, ecology, and conservation) are welcomed and should be sent to the editor. Today GNUSLETTER is published in English in electronic form and distributed widely to members and non-members, and to the IUCN SSC global conservation network. To be added to the distribution list please contact [email protected]. GNUSLETTER Editorial Board - David Mallon, ASG Co-Chair - Philippe Chardonnet, ASG Co-Chair ASG Program Office - Tania Gilbert, Marwell Wildife The Antelope Specialist Group Program Office is hosted and supported by Marwell Wildlife https://www.marwell.org.uk The designation of geographical entities in this report does not imply the expression of any opinion on the part of IUCN, the Species Survival Commission, or the Antelope Specialist Group concerning the legal status of any country, territory or area, or concerning the delimitation of any frontiers or boundaries. Views expressed in GNUSLETTER are those of the individual authors, Cover photo: Young female bushbuck (Tragelaphus scriptus), W National Park and Biosphere Reserve, Niger (© Daniel Cornélis) 2 GNUSLETTER Volume 37 Number 2 December 2020 FROM IUCN AND ASG……………………………………………………….