Clinical Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ticlopidine in Its Prodrug Form Is a Selective Inhibitor of Human Ntpdase1
Hindawi Publishing Corporation Mediators of Inflammation Volume 2014, Article ID 547480, 8 pages http://dx.doi.org/10.1155/2014/547480 Research Article Ticlopidine in Its Prodrug Form Is a Selective Inhibitor of Human NTPDase1 Joanna Lecka,1,2 Michel Fausther,1,2 Beat Künzli,3 and Jean Sévigny1,2 1 Departement´ de Microbiologie-Infectiologie et d’Immunologie, FacultedeM´ edecine,´ UniversiteLaval,Qu´ ebec,´ QC, Canada G1V 0A6 2 Centre de Recherche du CHU de Quebec,´ 2705 Boulevard Laurier, Local T1-49, Quebec,´ QC, Canada G1V 4G2 3 Department of Surgery, Klinikum Rechts der Isar, Technische Universitat¨ Munchen,¨ 81675 Munich, Germany Correspondence should be addressed to Jean Sevigny;´ [email protected] Received 12 May 2014; Accepted 21 July 2014; Published 11 August 2014 Academic Editor: Mireia Mart´ın-Satue´ Copyright © 2014 Joanna Lecka et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), like other ectonucleotidases, controls extracellular nucleotide levels and consequently their (patho)physiological responses such as in thrombosis, inflammation, and cancer. Selective NTPDase1 inhibitors would therefore be very useful. We previously observed that ticlopidine in its prodrug form, which does not affect P2 receptor activity, inhibited the recombinant form of human NTPDase1 ( =14M). Here we tested whether ticlopidine can be used as a selective inhibitor of NTPDase1. We confirmed that ticlopidine inhibits NTPDase1 in different forms and in different assays. The ADPase activity of intact HUVEC as well as of COS-7 cells transfected with human NTPDase1 was strongly inhibited by 100 M ticlopidine, 99 and 86%, respectively. -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

THE ROLE of CILOSTAZOL in ISCHEMIC STROKE
Since 1960 MedicineMedicine KoratKorat โรงพยาบาลมหาราชนครราชสีมา THE ROLE of CILOSTAZOL in ISCHEMIC STROKE PAWUT MEKAWICHAI MD DEPARTMENT of MEDICINE MAHARAT NAKHON RATCHASIMA HOSPITAL This presentation is supported by Thai Osuka CONTENT 1 Mechanism of cilostazol 2 Acute Ischemic Stroke (within 48h) 3 Secondary Prevention and Recurrence 4 Bleeding Complication CONTENT 1 Mechanism of cilostazol 2 Acute Ischemic Stroke (within 48h) 3 Secondary Prevention and Recurrence 4 Bleeding Complication Phosphodiesterase Inhibitor Increase cAMP → reduce platelet function Decrease cAMP → increase platelet function PDE enzyme change cAMP to 5-AMP Inhibit PDE enzyme → incrase cAMP → reduce platelet function Phosphodiesterase inhibitor Cilostazol Dipyridamole Classification of PDE Isozyme 9 family of PDE enzyme (PDE1-9) PDE-3 Site: platelet, heart, vascular SM, adipose tissue Inhibitor: Cilostazol PDE-5 Site: platelet, heart, vascular SM, corpus carvernosum Inhibitor: Dipyridamole, Sidenafil Distribution of PDE Isozyme Cell Platelet Heart Vascular Adipose SMC tissue Dominant III III III III Subordinate II V I II IV IV I II Inhibit PDE-3 antiplatelet action vasodilatation → headache ? inhibit vascular SMC proliferation tachycardia and palpitation increase HDL and decrease TG Which antiplatelet drug that can use in acute ischemic stroke? 1. ASA 81 mg 2. ASA 300 mg 3. Clopidogrel 4. Dipyridamole+ASA 5. Cilostazol CONTENT 1 Mechanism of cilostazol 2 Acute Ischemic Stroke (within 48h) 3 Secondary Prevention and Recurrence 4 Bleeding Complication ASPIRIN International Stroke Trial (IST) 1 ASA 300 mg-reduce mortality and recurrence in 2 wk) Chinese Acute Stroke Trial (CAST) 2 ASA 160 mg-reduce mortality and recurrence) reduce mortality rate 9/1,000 OR 0.92 (0.87-0.98) good functional outcome 7/1,000 OR 1.02 (1.01-1.04) increase severe bleeding (within 2-4 wk) 4/1,000 OR 1.69 (1.35-2.11) no evidence for difference dose of ASA 1.Lancet 1997; 349: 1569-81. -

A Comparative Study of Molecular Structure, Pka, Lipophilicity, Solubility, Absorption and Polar Surface Area of Some Antiplatelet Drugs
International Journal of Molecular Sciences Article A Comparative Study of Molecular Structure, pKa, Lipophilicity, Solubility, Absorption and Polar Surface Area of Some Antiplatelet Drugs Milan Remko 1,*, Anna Remková 2 and Ria Broer 3 1 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University in Bratislava, Odbojarov 10, SK-832 32 Bratislava, Slovakia 2 Department of Internal Medicine, Faculty of Medicine, Slovak Medical University, Limbová 12, SK–833 03 Bratislava, Slovakia; [email protected] 3 Department of Theoretical Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +421-2-5011-7291 Academic Editor: Michael Henein Received: 18 February 2016; Accepted: 11 March 2016; Published: 19 March 2016 Abstract: Theoretical chemistry methods have been used to study the molecular properties of antiplatelet agents (ticlopidine, clopidogrel, prasugrel, elinogrel, ticagrelor and cangrelor) and several thiol-containing active metabolites. The geometries and energies of most stable conformers of these drugs have been computed at the Becke3LYP/6-311++G(d,p) level of density functional theory. Computed dissociation constants show that the active metabolites of prodrugs (ticlopidine, clopidogrel and prasugrel) and drugs elinogrel and cangrelor are completely ionized at pH 7.4. Both ticagrelor and its active metabolite are present at pH = 7.4 in neutral undissociated form. The thienopyridine prodrugs ticlopidine, clopidogrel and prasugrel are lipophilic and insoluble in water. Their lipophilicity is very high (about 2.5–3.5 logP values). The polar surface area, with regard to the structurally-heterogeneous character of these antiplatelet drugs, is from very large interval of values of 3–255 Å2. -

Preventive Medication Program
PREVENTIVE MEDICATION PROGRAM Generics and Preferred Brands Drug List Starting July 1, 2021 Preventive medications are used to prevent certain conditions from developing, or to prevent a condition from coming back. These conditions include, but are not limited to, asthma, depression, diabetes, heart attack, high blood pressure, high cholesterol, osteoporosis, prenatal nutrient deficiency and stroke. About this drug list. Your cost-share for preventive generic and This is a list of the most commonly prescribed generic preferred brand medications. and preferred brand medications that are part of Not all plans offer the same cost-share for their Cigna’s preventive medication program as of July preventive medication program. For example, some 1, 2021.1,2 This drug list is updated often so it isn’t a plans may require you to pay a copay, coinsurance complete list of medications. Also, your specific plan’s and/or deductible for preventive generic and preferred preventive medication program may not include all brand medications; other plans may not. of these medications and/or conditions. Log in to the Log into the myCigna App or myCigna.com and use myCigna® App or myCigna.com, or check your plan the Price a Medication tool to see how much your materials, to see all of the medications included in your medication may cost you at the different pharmacies in plan’s preventive medication program and how much your plan’s network.3 they cost. Here’s some helpful information about this drug list: › Medications are listed alphabetically by condition. › Brand-name medications are capitalized and generic medications are lowercase. -

Wo 2010/075090 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 1 July 2010 (01.07.2010) WO 2010/075090 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 409/14 (2006.01) A61K 31/7028 (2006.01) kind of national protection available): AE, AG, AL, AM, C07D 409/12 (2006.01) A61P 11/06 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2009/068073 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 December 2009 (15.12.2009) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, (26) Publication Language: English TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/122,478 15 December 2008 (15.12.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): AUS- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, PEX PHARMACEUTICALS, INC. -

Preventive Generic Drugs Listing
2017 PREVENTIVE DRUG LIST Preventive medications are used for the prevention of conditions such as high blood pressure, high cholesterol, diabetes, asthma, osteoporosis, heart attack, stroke and prenatal nutrient deficiency. You may not have to pay a copay, a coinsurance (the percentage you pay after you meet your deductible) and/or a deductible (the amount you pay before your plan starts to pay) for preventive medications. Please check your plan’s prescription drug list and This list is subject to change and may not include all plan documents to understand how your plan covers preventive medications that your plan covers. Please preventive medications. You can refer to myCigna.com refer to your plan’s prescription drug list for a complete for a complete and up-to-date drug listing for your plan. and up-to-date drug listing. You can also use the Prescription Drug Price Quote tool on myCigna.com to view and compare drug prices. The following is a list of generic and brand-name If you have questions preventive medications, arranged by type of condition. Please call the toll-free number on the back of Talk with your doctor when choosing the preventive your Cigna ID card. We’re here to help. medication that is right for you. Also talk with your doctor about available generic preventive medications (listed as uncapitalized medications and/or in parentheses). Generic medications have the same active ingredients, dosage form, quality and strength, as their brand-name counterparts and may provide additional savings for you. Offered by: Cigna Health and Life Insurance Company, Connecticut General Life Insurance Company, or their affiliates. -

In Vitro Modulation of Cisplatin Accumulation in Human Ovarian Carcinoma Cells by Pharmacologic Alteration of Microtubules
In vitro modulation of cisplatin accumulation in human ovarian carcinoma cells by pharmacologic alteration of microtubules. R D Christen, … , D R Shalinsky, S B Howell J Clin Invest. 1993;92(1):431-440. https://doi.org/10.1172/JCI116585. Research Article We have previously shown that forskolin and 3-isobutyl-1-methylxanthine (IBMX) increased accumulation of cisplatin (DDP) in DDP-sensitive 2008 human ovarian carcinoma cells in proportion to their ability to increase cAMP. Since the major function of cAMP is to activate protein kinase A, it was conjectured that the stimulation of DDP accumulation was mediated by a protein kinase A substrate. We now show that exposure of 2008 cells to forskolin resulted in phosphorylation of a prominent 52-kD membrane protein. Microsequencing of the band demonstrated it to be human beta-tubulin. Similarly, pretreatment of 2008 cells with the microtubule stabilizing drug taxol increased platinum accumulation in a dose-dependent manner. In 11-fold DDP-resistant 2008/C13*5.25 cells, decreased DDP accumulation was associated with enhanced spontaneous formation of microtubule bundles and decreased expression of beta-tubulin and the tubulin-associated p53 antioncogene relative to 2008 cells. 2008/C13*5.25 cells had altered sensitivity to tubulin- binding drugs, being hypersensitive to taxol and cross-resistant to colchicine. We conclude that pharmacologic alterations of tubulin enhance accumulation of DDP, and that the DDP-resistant phenotype in 2008/C13*5.25 cells is associated with tubulin abnormalities. Find the latest version: https://jci.me/116585/pdf In Vitro Modulation of Cisplatin Accumulation in Human Ovarian Carcinoma Cells by Pharmacologic Alteration of Microtubules Randolph D. -

MEDICINE to TREAT: HEART DISEASES Antiplatelet Agents Aspirin Dipyridamole Clopidogrel Ticlopidine 1. What Are These Medicines U
PATIENT INFORMATION LEAFLET MEDICINE TO TREAT: HEART DISEASES Antiplatelet Agents Aspirin Dipyridamole Clopidogrel Ticlopidine 1. What are these medicines used for? Medicine Purpose of medicine Brand Names This medicine makes blood less sticky to reduce the chance of blood forming Aspirin Cardiprin® clots which will lead to stroke or heart Disprin® attack. This medicine makes the blood less Dipyridamole sticky to reduce the chance of stroke. It Persantin® is used together with aspirin. These medicines make your blood less Clopidogrel (Plavix®) Clopidogrel sticky to reduce chance of stroke or Ticlopidine (Ticlid®), Ticlopidine heart attack. They can be used alone or together with aspirin. 2. How should I take the medicines? • Do not stop taking your medicines without checking with your doctors. • If you miss a dose, take the missed dose as soon as you remember. If it is almost time for your next dose, take only the usual dose. Do not double your dose or use extra medicine to make up for the missed dose. • You should take your medicine after a meal to prevent stomach upset. • How you take aspirin depends on the brand and type of aspirin the doctor gives you: o For tablets which can be chewed: Take with food or glass of water or milk These tablets may be chewed or swallowed whole The solution may also be used as a gargle. Please check with your pharmacist on how to use it as a gargle. o For tablets which enteric coated: Enteric coated forms of aspirin reduce stomach upset that is caused by the medicine Take with a glass of water after food. -

Pyridylisatogen Tosylate of ATP-Responses at a Recombinant P2y1 Purinoceptor 1B.F
British Journal of Pharmacology (I996) 117, 1111 1118 1996 Stockton Press All rights reserved 0007-1188/96 $12.00 0 Potentiation by 2,2'-pyridylisatogen tosylate of ATP-responses at a recombinant P2y1 purinoceptor 1B.F. King, *C. Dacquet, A.U. Ziganshin, tD.F. Weetman, G. Burnstock, *P.M. Vanhoutte & *M. Spedding Department ofAnatomy and Developmental Biology, University College London, Gower Street, London WClE 6BT; *Institute de Recherches Servier, 125 Chemin de Ronde, Croissy sur Seine, 72890, France and tDepartment of Pharmacology, University of Sunderland, Tyne and Wear SRI 3SD 1 2,2'-Pyridylisatogen tosylate (PIT) has been reported to be an irreversible antagonist of responses to adenosine 5'-triphosphate (ATP) at metabotropic purinoceptors (of the P2Y family) in some smooth muscles. When a recombinant P2Y purinoceptor (derived from chick brain) is expressed in Xenopus oocytes, ATP and 2-methylthioATP (2-MeSATP) evoke calcium-activated chloride currents (IC1,ca) in a concentration-dependent manner. The effects of PIT on these agonist responses were examined at this cloned P2Y purinoceptor. 2 PIT (0.1-100 rM) failed to stimulate P2y, purinoceptors directly but, over a narrow concentration range (0.1-3 tM), caused a time-dependent potentiation (2-5 fold) of responses to ATP. The potentiation of ATP-responses by PIT was not caused by inhibition of oocyte ecto-ATPase. At high concentrations (3-100 gM), PIT irreversibly inhibited responses to ATP with a IC_0 value of 13 +9 gM (pKB= 4.88 + 0.22; n = 3). PIT failed to potentiate inward currents evoked by 2-MeSATP and only inhibited the responses to this agonist in an irreversible manner. -

Use of Antiplatelet Therapies During Primary Percutaneous Coronary Intervention for Acute Myocardial Infarction
REVIEW Use of antiplatelet therapies during primary percutaneous coronary intervention for acute myocardial infarction Inhibition of platelet function is necessary to achieve successful and long-lasting primary percutaneous coronary intervention (PCI) for acute myocardial infarction. For many years, antiplatelet therapy in the setting of primary PCI consisted of two drugs that inhibit platelet activation (aspirin and clopidogrel) and an intravenous blocker of platelet aggregation (abciximab). The development of new, more potent oral antiplatelet drugs (prasugrel and ticagrelor) as well as new data on clopidogrel dosing regimens limited the use of abciximab after pretreatment with aspirin and clopidogrel. Thus, intracoronary administration of abciximab and the use of small molecule glycoprotein IIb/IIIa blockers (tirofiban or eptifibatide) challenge the contemporary schemes of antiplatelet treatment in primary PCI. We review recently published data with particular attention on patients and drug characteristics and propose an update of existing recommendations. 1 KEYWORDS: acute myocardial infarction n antiplatelet therapy n aspirin n cilostazol Łukasz A Małek n clopidogrel n glycoprotein IIb/IIIa blockers n prasugrel n primary percutaneous & Adam Witkowski†1 coronary intervention n ticagrelor 1Department of Interventional Cardiology & Angiology, Institute of Cardiology, Alpejska 42, Rupture of the vulnerable atherosclerotic many years, antiplatelet therapy in the setting 04‑628 Warsaw, Poland plaque in the coronary artery wall leading of primary PCI included two oral antiplate- †Author for correspondence: Tel.: +48 22 343 4127 to activation and aggregation of platelets to let drugs, which irreversibly block platelet Fax: +48 22 613 3819 form an artery occluding thrombus is the most activation (aspirin and clopidogrel) together [email protected] frequent cause of acute myocardial infarc- with an inhibition of platelet aggregation by tion (AMI) [1–3]. -
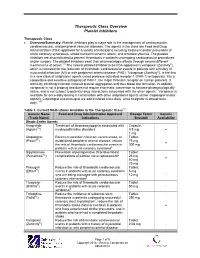
Therapeutic Class Overview Platelet Inhibitors
Therapeutic Class Overview Platelet Inhibitors Therapeutic Class • Overview/Summary: Platelet inhibitors play a major role in the management of cardiovascular, cerebrovascular, and peripheral vascular diseases. The agents in the class are Food and Drug Administration (FDA)-approved for a variety of indications including treatment and/or prevention of acute coronary syndromes, stroke/transient ischemic attack, and thrombocythemia. The platelet inhibitors are also indicated to prevent thrombosis in patients undergoing cardiovascular procedures and/or surgery. The platelet inhibitors exert their pharmacologic effects through several different mechanisms of action.1-8 The newest platelet inhibitor to be FDA-approved is vorapaxar (Zontivity®), which is indicated for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or with peripheral arterial disease (PAD).7 Vorapaxar (Zontivity®), is the first in a new class of antiplatelet agents called protease-activated receptor-1 (PAR-1) antagonists. It is a competitive and selective antagonist of PAR-1, the major thrombin receptor on human platelets. It works by inhibiting thrombin-induced platelet aggregation and thus blood clot formation. In addition, vorapaxar is not a prodrug and does not require enzymatic conversion to become pharmacologically active, and is not subject to potential drug interactions associated with the other agents.7 Vorapaxar is available for once-daily dosing in combination with other antiplatelet agents (either clopidogrel