G Protein-Coupled Receptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands
The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands Clare Louise Wishart Submitted in accordance with the requirements for the degree of Doctor of Philosophy of Science University of Leeds School of Biomedical Sciences Faculty of Biological Sciences September 2013 I Intellectual Property and Publication Statements The candidate confirms that the work submitted is her own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Clare Louise Wishart to be identified as Author of this work has been asserted by her in accordance with the Copyright, Designs and Patents Act 1988. © 2013 The University of Leeds and Clare Louise Wishart. II Acknowledgments Firstly I would like to offer my sincerest thanks and gratitude to my supervisor, Dr. Dan Donnelly, who has been nothing but encouraging and engaging from day one. I have thoroughly enjoyed every moment of working alongside him and learning from his guidance and wisdom. My thanks go to my academic assessor Professor Paul Milner whom I have known for several years, and during my time at the University of Leeds he has offered me invaluable advice and inspiration. Additionally I would like to thank my academic project advisor Dr. Michael Harrison for his friendship, help and advice. I would like to thank Dr. Rosalind Mann and Dr. Elsayed Nasr for welcoming me into the lab as a new PhD student and sharing their experimental techniques with me, these techniques have helped me no end in my time as a research student. -

Emerging Evidence for a Central Epinephrine-Innervated A1- Adrenergic System That Regulates Behavioral Activation and Is Impaired in Depression
Neuropsychopharmacology (2003) 28, 1387–1399 & 2003 Nature Publishing Group All rights reserved 0893-133X/03 $25.00 www.neuropsychopharmacology.org Perspective Emerging Evidence for a Central Epinephrine-Innervated a1- Adrenergic System that Regulates Behavioral Activation and is Impaired in Depression ,1 1 1 1 1 Eric A Stone* , Yan Lin , Helen Rosengarten , H Kenneth Kramer and David Quartermain 1Departments of Psychiatry and Neurology, New York University School of Medicine, New York, NY, USA Currently, most basic and clinical research on depression is focused on either central serotonergic, noradrenergic, or dopaminergic neurotransmission as affected by various etiological and predisposing factors. Recent evidence suggests that there is another system that consists of a subset of brain a1B-adrenoceptors innervated primarily by brain epinephrine (EPI) that potentially modulates the above three monoamine systems in parallel and plays a critical role in depression. The present review covers the evidence for this system and includes findings that brain a -adrenoceptors are instrumental in behavioral activation, are located near the major monoamine cell groups 1 or target areas, receive EPI as their neurotransmitter, are impaired or inhibited in depressed patients or after stress in animal models, and a are restored by a number of antidepressants. This ‘EPI- 1 system’ may therefore represent a new target system for this disorder. Neuropsychopharmacology (2003) 28, 1387–1399, advance online publication, 18 June 2003; doi:10.1038/sj.npp.1300222 Keywords: a1-adrenoceptors; epinephrine; motor activity; depression; inactivity INTRODUCTION monoaminergic systems. This new system appears to be impaired during stress and depression and thus may Depressive illness is currently believed to result from represent a new target for this disorder. -
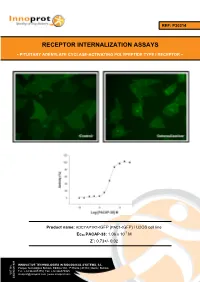
Receptor Internalization Assays
REF: P30214 RECEPTOR INTERNALIZATION ASSAYS - PITUITARY ADENYLATE CYCLASE-ACTIVATING POLYPEPTIDE TYPE I RECEPTOR - Product name: ADCYAP1R1-tGFP (PAC1-tGFP) / U2OS cell line -7 Ec50 PACAP-38: 1.06 x 10 M Z´: 0.73+/- 0.02 INNOVATIVE TECHNOLOGIES IN BIOLOGICAL SYSTEMS, S.L. Parque Tecnológico Bizkaia, Edifício 502, 1ª Planta | 48160 | Derio | Bizkaia Tel.: +34 944005355 | Fax: +34 946579925 VAT No. [email protected] | www.innoprot.com ESB95481909 Product Name: ADCYAP1R1-tGFP_U2OS Reference: P30214 Rep. Official Full Name: Pituitary adenylate cyclase- activating polypeptide type I receptor DNA Accession Number: Gene Bank AY366498 Host Cell: U2OS References: P30214: 2 vials of 3 x 106 proliferative cells P30214-DA: 1 vial of 2 x 106 division-arrested cells Storage: Liquid Nitrogen Assay Briefly description About ADCYAP1R1 Each vial of ADCYAP1R1 Internalization Assay Pituitary adenylate cyclase-activating Cell Line contains U2OS cells stably expressing polypeptide type I receptor, also known as human Pituitary adenylate cyclase-activating PAC1 is a protein that in humans is encoded by polypeptide type I receptor tagged in the N- the ADCYAP1R1 gene. ADCYAP1R1 is a terminus with tGFP protein. membrane-associated protein and shares significant homology with members of the Innoprot’s ADCYAP1R1-tGFP Internalization glucagon/secretin receptor family. This receptor Assay Cell Line has been designed to assay binds pituitary adenylate cyclase activating potential agonists/ antagonists against peptide (PACAP) mediating several biological ADCYAP1R1, modulating its activation and the activities and it is positively coupled to following redistribution process inside the cells. adenylate cyclase. This cell line will allow the image analysis of the stimuli induced by the compounds. -

Metabotropic Glutamate Receptors
mGluR Metabotropic glutamate receptors mGluR (metabotropic glutamate receptor) is a type of glutamate receptor that are active through an indirect metabotropic process. They are members of thegroup C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatoryneurotransmitter. The mGluRs perform a variety of functions in the central and peripheral nervous systems: mGluRs are involved in learning, memory, anxiety, and the perception of pain. mGluRs are found in pre- and postsynaptic neurons in synapses of the hippocampus, cerebellum, and the cerebral cortex, as well as other parts of the brain and in peripheral tissues. Eight different types of mGluRs, labeled mGluR1 to mGluR8, are divided into groups I, II, and III. Receptor types are grouped based on receptor structure and physiological activity. www.MedChemExpress.com 1 mGluR Agonists, Antagonists, Inhibitors, Modulators & Activators (-)-Camphoric acid (1R,2S)-VU0155041 Cat. No.: HY-122808 Cat. No.: HY-14417A (-)-Camphoric acid is the less active enantiomer (1R,2S)-VU0155041, Cis regioisomer of VU0155041, is of Camphoric acid. Camphoric acid stimulates a partial mGluR4 agonist with an EC50 of 2.35 osteoblast differentiation and induces μM. glutamate receptor expression. Camphoric acid also significantly induced the activation of NF-κB and AP-1. Purity: ≥98.0% Purity: ≥98.0% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10 mM × 1 mL, 100 mg Size: 10 mM × 1 mL, 5 mg, 10 mg, 25 mg (2R,4R)-APDC (R)-ADX-47273 Cat. No.: HY-102091 Cat. No.: HY-13058B (2R,4R)-APDC is a selective group II metabotropic (R)-ADX-47273 is a potent mGluR5 positive glutamate receptors (mGluRs) agonist. -

Synthetic Nanobodies As Angiotensin Receptor Blockers
Synthetic nanobodies as angiotensin receptor blockers Conor McMahona,1, Dean P. Stausb,c,1, Laura M. Winglerb,c,1,2, Jialu Wangc, Meredith A. Skibaa, Matthias Elgetid,e, Wayne L. Hubbelld,e, Howard A. Rockmanc,f, Andrew C. Krusea,3, and Robert J. Lefkowitzb,c,g,3 aDepartment of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115; bHoward Hughes Medical Institute, Duke University Medical Center, Durham, NC 27710; cDepartment of Medicine, Duke University Medical Center, Durham, NC 27710; dJules Stein Eye Institute, University of California, Los Angeles, CA 90095; eDepartment of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095; fDepartment of Cell Biology, Duke University Medical Center, Durham, NC 27710; and gDepartment of Biochemistry, Duke University Medical Center, Durham, NC 27710 Edited by K. Christopher Garcia, Stanford University, Stanford, CA, and approved July 13, 2020 (received for review May 6, 2020) There is considerable interest in developing antibodies as functional a need for more broadly applicable methodologies to discover modulators of G protein-coupled receptor (GPCR) signaling for both antibody fragments explicitly directed to the membrane- therapeutic and research applications. However, there are few an- embedded domains with limited surface exposure. tibody ligands targeting GPCRs outside of the chemokine receptor The angiotensin II type 1 receptor (AT1R) is a GPCR that group. GPCRs are challenging targets for conventional antibody dis- exemplifies the opportunities and the challenges surrounding an- covery methods, as many are highly conserved across species, are tibody drug development. Both the endogenous peptide agonist of biochemically unstable upon purification, and possess deeply buried the AT1R (angiotensin II) and small-molecule inhibitors (angio- ligand-binding sites. -

The Impact of the Nonpeptide Corticotropin-Releasing Hormone Antagonist Antalarmin on Behavioral and Endocrine Responses to Stress*
0013-7227/99/$03.00/0 Vol. 140, No. 1 Endocrinology Printed in U.S.A. Copyright © 1999 by The Endocrine Society The Impact of the Nonpeptide Corticotropin-Releasing Hormone Antagonist Antalarmin on Behavioral and Endocrine Responses to Stress* TERRENCE DEAK, KIEN T. NGUYEN, ANDREA L. EHRLICH, LINDA R. WATKINS, ROBERT L. SPENCER, STEVEN F. MAIER, JULIO LICINIO, MA-LI WONG, GEORGE P. CHROUSOS, ELIZABETH WEBSTER, AND PHILIP W. GOLD Department of Psychology (T.D., K.T.N., A.L.E., L.R.W., R.L.S., S.F.M.), University of Colorado, Boulder, Colorado 80309-0345; Clinical Neuroendocrinology Branch (J.L., M.-L.W., P.W.G.), National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland 20892-1284; and Developmental Neuroendocrinology Branch (G.P.C., E.W.), National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland 20892-1284 ABSTRACT Furthermore, because rats previously exposed to inescapable shock The nonpeptide CRH antagonist antalarmin has been shown to (IS; 100 shocks, 1.6 mA, 5 sec each), demonstrate enhanced fear block both behavioral and endocrine responses to CRH. However, it’s conditioning, we investigated whether this effect would be blocked by potential activity in blunting behavioral and endocrine sequelae of antalarmin. Antalarmin (20 mg/kgz2 ml ip) impaired both the induc- stressor exposure has not been assessed. Because antagonism of cen- tion and expression of conditioned fear. In addition, antalarmin tral CRH by a-helical CRH attenuates conditioned fear responses, we blocked the enhancement of fear conditioning produced by prior ex- sought to test antalarmin in this regard. -

Adverse Effects of Stress on Drug Addiction
Making a bad thing worse: adverse effects of stress on drug addiction Jessica N. Cleck, Julie A. Blendy J Clin Invest. 2008;118(2):454-461. https://doi.org/10.1172/JCI33946. Review Series Sustained exposure to various psychological stressors can exacerbate neuropsychiatric disorders, including drug addiction. Addiction is a chronic brain disease in which individuals cannot control their need for drugs, despite negative health and social consequences. The brains of addicted individuals are altered and respond very differently to stress than those of individuals who are not addicted. In this Review, we highlight some of the common effects of stress and drugs of abuse throughout the addiction cycle. We also discuss both animal and human studies that suggest treating the stress- related aspects of drug addiction is likely to be an important contributing factor to a long-lasting recovery from this disorder. Find the latest version: https://jci.me/33946/pdf Review series Making a bad thing worse: adverse effects of stress on drug addiction Jessica N. Cleck and Julie A. Blendy Department of Pharmacology, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. Sustained exposure to various psychological stressors can exacerbate neuropsychiatric disorders, including drug addiction. Addiction is a chronic brain disease in which individuals cannot control their need for drugs, despite negative health and social consequences. The brains of addicted individuals are altered and respond very differently to stress than those of individuals who are not addicted. In this Review, we highlight some of the common effects of stress and drugs of abuse throughout the addiction cycle. -

Extracellular Adenosine Triphosphate and Adenosine in Cancer
Oncogene (2010) 29, 5346–5358 & 2010 Macmillan Publishers Limited All rights reserved 0950-9232/10 www.nature.com/onc REVIEW Extracellular adenosine triphosphate and adenosine in cancer J Stagg and MJ Smyth Cancer Immunology Program, Sir Donald and Lady Trescowthick Laboratories, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia Adenosine triphosphate (ATP) is actively released in the mulated the hypothesis of purinergic neurotransmission extracellular environment in response to tissue damage (Burnstock, 1972). Burnstock’s hypothesis that ATP and cellular stress. Through the activation of P2X and could be released by cells to perform intercellular P2Y receptors, extracellular ATP enhances tissue repair, signaling was initially met with skepticism, as it seemed promotes the recruitment of immune phagocytes and unlikely that a molecule that acts as an intracellular dendritic cells, and acts as a co-activator of NLR family, source of energy would also function as an extracellular pyrin domain-containing 3 (NLRP3) inflammasomes. messenger. Nevertheless, Burnstock pursued his work The conversion of extracellular ATP to adenosine, in and, together with Che Su and John Bevan, reported contrast, essentially through the enzymatic activity of the that ATP was also released from sympathetic nerves ecto-nucleotidases CD39 and CD73, acts as a negative- during stimulation (Su et al., 1971). Three decades later, feedback mechanism to prevent excessive immune responses. following the cloning and characterization of ATP and Here we review the effects of extracellular ATP and adenosine adenosine cell surface receptors, purinergic signaling is a on tumorigenesis. First, we summarize the functions of well-established concept and constitutes an expanding extracellular ATP and adenosine in the context of tumor field of research in health and disease, including cancer immunity. -

P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases
International Journal of Molecular Sciences Review P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases Derek Strassheim 1, Alexander Verin 2, Robert Batori 2 , Hala Nijmeh 3, Nana Burns 1, Anita Kovacs-Kasa 2, Nagavedi S. Umapathy 4, Janavi Kotamarthi 5, Yash S. Gokhale 5, Vijaya Karoor 1, Kurt R. Stenmark 1,3 and Evgenia Gerasimovskaya 1,3,* 1 The Department of Medicine Cardiovascular and Pulmonary Research Laboratory, University of Colorado Denver, Aurora, CO 80045, USA; [email protected] (D.S.); [email protected] (N.B.); [email protected] (V.K.); [email protected] (K.R.S.) 2 Vascular Biology Center, Augusta University, Augusta, GA 30912, USA; [email protected] (A.V.); [email protected] (R.B.); [email protected] (A.K.-K.) 3 The Department of Pediatrics, Division of Critical Care Medicine, University of Colorado Denver, Aurora, CO 80045, USA; [email protected] 4 Center for Blood Disorders, Augusta University, Augusta, GA 30912, USA; [email protected] 5 The Department of BioMedical Engineering, University of Wisconsin, Madison, WI 53706, USA; [email protected] (J.K.); [email protected] (Y.S.G.) * Correspondence: [email protected]; Tel.: +1-303-724-5614 Received: 25 August 2020; Accepted: 15 September 2020; Published: 18 September 2020 Abstract: Purinergic G-protein-coupled receptors are ancient and the most abundant group of G-protein-coupled receptors (GPCRs). The wide distribution of purinergic receptors in the cardiovascular system, together with the expression of multiple receptor subtypes in endothelial cells (ECs) and other vascular cells demonstrates the physiological importance of the purinergic signaling system in the regulation of the cardiovascular system. -

A Plant-Based Meal Increases Gastrointestinal Hormones
nutrients Article A Plant-Based Meal Increases Gastrointestinal Hormones and Satiety More Than an Energy- and Macronutrient-Matched Processed-Meat Meal in T2D, Obese, and Healthy Men: A Three-Group Randomized Crossover Study Marta Klementova 1, Lenka Thieme 1 , Martin Haluzik 1, Renata Pavlovicova 1, Martin Hill 2, Terezie Pelikanova 1 and Hana Kahleova 1,3,* 1 Institute for Clinical and Experimental Medicine, 140 21 Prague, Czech Republic; [email protected] (M.K.); [email protected] (L.T.); [email protected] (M.H.); [email protected] (R.P.); [email protected] (T.P.) 2 Institute of Endocrinology, 113 94 Prague, Czech Republic; [email protected] 3 Physicians Committee for Responsible Medicine, Washington, DC 20016, USA * Correspondence: [email protected]; Tel.: +1-202-527-7379 Received: 6 December 2018; Accepted: 9 January 2019; Published: 12 January 2019 Abstract: Gastrointestinal hormones are involved in regulation of glucose metabolism and satiety. We tested the acute effect of meal composition on these hormones in three population groups. A randomized crossover design was used to examine the effects of two energy- and macronutrient-matched meals: a processed-meat and cheese (M-meal) and a vegan meal with tofu (V-meal) on gastrointestinal hormones, and satiety in men with type 2 diabetes (T2D, n = 20), obese men (O, n = 20), and healthy men (H, n = 20). Plasma concentrations of glucagon-like peptide -1 (GLP-1), amylin, and peptide YY (PYY) were determined at 0, 30, 60, 120 and 180 min. Visual analogue scale was used to assess satiety. We used repeated-measures Analysis of variance (ANOVA) for statistical analysis. -

Β2‑Adrenergic Receptor Functionality and Genotype in Two Different Models of Chronic Inflammatory Disease: Liver Cirrhosis and Osteoarthritis
MOLECULAR MEDICINE REPORTS 17: 7987-7995, 2018 β2‑adrenergic receptor functionality and genotype in two different models of chronic inflammatory disease: Liver cirrhosis and osteoarthritis REYES ROCA1, PABLO ESTEBAN1, PEDRO ZAPATER2,3, MARÍA-DEL-MAR INDA4, ANNA LUCIA CONTE1, LAURA GÓMEZ-ESCOLAR5, HELENA MARTÍNEZ6, JOSÉ F. HORGA3, JOSÉ M. PALAZON5 and ANA M. PEIRÓ3,4 1Occupational Observatory, Miguel Hernández University (UMH) of Elche, 03202 Elche; 2CIBERehd, Carlos III Health Institute, 28029 Madrid; 3Clinical Pharmacology, General Hospital of Alicante; 4Neuropharmacology on Pain (NED) Research Group, ISABIAL-FISABIO, General Hospital of Alicante; 5Liver Unit, General Hospital of Alicante, 03010 Alicante; 6Clinical R&D Area, Bioiberica S.A., 08029 Barcelona, Spain Received June 12, 2017; Accepted September 28, 2017 DOI: 10.3892/mmr.2018.8820 Abstract. The present study was designed to investigate the Introduction functional status of β2 adrenoceptors (β2AR) in two models of chronic inflammatory disease: Liver cirrhosis (LC) and The role of the sympathetic nervous system (SNS) in inflam- osteoarthritis (OA). The β2AR gene contains three single mation is still not completely understood, although it is well nucleotide polymorphisms at amino acid positions 16, 27 and known that disturbed interaction between both contributes to 164. The aim of the present study was to investigate the poten- pathogenic chronic inflammatory diseases (1,2). Evidence for tial influence of lymphocyte β2AR receptor functionality and the possibility of such interaction have been reinforced since genotype in LC and OA patients. Blood samples from cirrhotic the discovery of the expression of beta-2-adrenergic receptor patients (n=52, hepatic venous pressure gradient 13±4 mmHg, (β2AR) on T and B lymphocytes, macrophages, natural killer CHILD 7±2 and MELD 11±4 scores), OA patients (n=30, 84% cells and neutrophils (3-6). -

Acute Restraint Stress Induces Cholecystokinin Release Via Enteric
Neuropeptides 73 (2019) 71–77 Contents lists available at ScienceDirect Neuropeptides journal homepage: www.elsevier.com/locate/npep Acute restraint stress induces cholecystokinin release via enteric apelin T ⁎ Mehmet Bülbüla, , Osman Sinena, Onur Bayramoğlua, Gökhan Akkoyunlub a Department of Physiology, Akdeniz University, Faculty of Medicine, Antalya, Turkey b Department of Histology and Embryology, Akdeniz University, Faculty of Medicine, Antalya, Turkey ARTICLE INFO ABSTRACT Keywords: Stress increases the apelin content in gut, while exogenous peripheral apelin has been shown to induce chole- Apelin cystokinin (CCK) release. The present study was designed to elucidate (i) the effect of acute stress on enteric Restraint stress production of apelin and CCK, (ii) the role of APJ receptors in apelin-induced CCK release depending on the Cholecystokinin nutritional status. CCK levels were assayed in portal vein blood samples obtained from stressed (ARS) and non- APJ receptor stressed (NS) rats previously injected with APJ receptor antagonist F13A or vehicle. Duodenal expressions of Fasting apelin, CCK and APJ receptor were detected by immunohistochemistry. ARS increased the CCK release which was abolished by selective APJ receptor antagonist F13A. The stimulatory effect of ARS on CCK production was only observed in rats fed ad-libitum. Apelin and CCK expressions were upregulated by ARS. In addition to the duodenal I cells, APJ receptor was also detected in CCK-producing myenteric neurons. Enteric apelin appears to regulate the stress-induced changes in GI functions through CCK. Therefore, apelin/APJ receptor systems seem to be a therapeutic target for the treatment of stress-related gastrointestinal disorders. 1. Introduction for APJ in rodents (De Mota et al., 2000; Medhurst et al., 2003).