Toxicological Profile for Uranium, September 1999
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
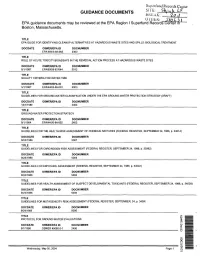
List of Guidance Documents for Shpack Landfill Record
Super fund Records Center GUIDANCE DOCUMENTS bKiS.K- ' 2 "7 OHihR: 2tofe *, EPA guidance documents may be reviewed at the EPA Region I Superfund Records Center inl Boston, Massachusetts. TITLE EPA GUIDE FOR IDENTIFYING CLEANUP ALTERNATIVES AT HAZARDOUS-WASTE SITES AND SPILLS: BIOLOGICAL TREATMENT DOCDATE OSWER/EPA ID DOCNUMBER EPA-600/3-83-063 2303 TITLE ROLE OF ACUTE TOXICITY BIOASSAYS IN THE REMEDIAL ACTION PROCESS AT HAZARDOUS WASTE SITES DOCDATE OSWER/EPA ID DOCNUMBER 8/1/1987 EPA/600/8-87/044 5012 TITLE QUALITY CRITERIA FOR WATER 1986 DOCDATE OSWER/EPA ID DOCNUMBER 5/1/1987 EPA/440/5-86-001 4003 TITLE GUIDELINES FOR GROUND-WATER CLASSIFICATION UNDER THE EPA GROUND-WATER PROTECTION STRATEGY (DRAFT) DOCDATE OSWER/EPA ID DOCNUMBER 12/1/1986 2404 TITLE GROUND-WATER PROTECTION STRATEGY DOCDATE OSWER/EPA ID DOCNUMBER 8/1/1984 EPA/440/6-84-002 2403 TITLE GUIDELINES FOR THE HEALTH RISK ASSESSMENT OF CHEMICAL MIXTURES (FEDERAL REGISTER, SEPTEMBER 24, 1986, p. 34014) DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5007 TITLE GUIDELINES FOR CARCINOGEN RISK ASSESSMENT (FEDERAL REGISTER, SEPTEMBER 24, 1986, p. 33992) DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5003 TITLE GUIDELINES FOR EXPOSURE ASSESSMENT (FEDERAL REGISTER, SEPTEMBER 24, 1986, p. 34042) DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5004 TITLE GUIDELINES FOR HEALTH ASSESSMENT OF SUSPECT DEVELOPMENTAL TOXICANTS (FEDERAL REGISTER, SEPTEMBER 24,1986, p. 34028) DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5005 TITLE GUIDELINES FOR MUTAGENICITY RISK ASSESSMENT (FEDERAL REGISTER, SEPTEMBER, 24, p. 34006 DOCDATE OSWER/EPA ID DOCNUMBER 9/24/1986 5006 TITLE PROTOCOL FOR GROUND-WATER EVALUATIONS DOCDATE OSWER/EPA ID DOCNUMBER 9/1/1986 OSWER #9080.0-1 2406 Wednesday, May 05, 2004 Page 1 GUIDANCE DOCUMENTS EPA guidance documents may be reviewed at the EPA Region I Superfund Records Center in Boston, Massachusetts. -

Report to the Legislature: Indoor Air Pollution in California
California Environmental Protection Agency Air Resources Board Report to the California Legislature INDOOR AIR POLLUTION IN CALIFORNIA A report submitted by: California Air Resources Board July, 2005 Pursuant to Health and Safety Code § 39930 (Assembly Bill 1173, Keeley, 2002) Arnold Schwarzenegger Governor Indoor Air Pollution in California July, 2005 ii Indoor Air Pollution in California July, 2005 ACKNOWLEDGEMENTS This report was prepared with the able and dedicated support from Jacqueline Cummins, Marisa Bolander, Jeania Delaney, Elizabeth Byers, and Heather Choi. We appreciate the valuable input received from the following groups: • Many government agency representatives who provided information and thoughtful comments on draft reports, especially Jed Waldman, Sandy McNeel, Janet Macher, Feng Tsai, and Elizabeth Katz, Department of Health Services; Richard Lam and Bob Blaisdell, Office of Environmental Health Hazard Assessment; Deborah Gold and Bob Nakamura, Cal/OSHA; Bill Pennington and Bruce Maeda, California Energy Commission; Dana Papke and Kathy Frevert, California Integrated Waste Management Board; Randy Segawa, and Madeline Brattesani, Department of Pesticide Regulation; and many others. • Bill Fisk, Lawrence Berkeley National Laboratory, for assistance in assessing the costs of indoor pollution. • Susan Lum, ARB, project website management, and Chris Jakober, for general technical assistance. • Stakeholders from the public and private sectors, who attended the public workshops and shared their experiences and suggestions -

In Situ Leach (ISL) Mining of Uranium
In Situ Leach (ISL) Mining of Uranium (June 2009) l Most uranium mining in the USA and Kazakhstan is now by in situ leach methods, also known as in situ recovery (ISR). l In USA ISL is seen as the most cost effective and environmentally acceptable method of mining, and Australian experience supports this. l Australia's first ISL uranium mine is Beverley, which started operation late in 2000. The proposal for Honeymoon has government approval and it is expected to be operating in 2008. Conventional mining involves removing mineralised rock (ore) from the ground, breaking it up and treating it to remove the minerals being sought. In situ leaching (ISL), also known as solution mining, or in situ recovery (ISR) in North America, involves leaving the ore where it is in the ground, and recovering the minerals from it by dissolving them and pumping the pregnant solution to the surface where the minerals can be recovered. Consequently there is little surface disturbance and no tailings or waste rock generated. However, the orebody needs to be permeable to the liquids used, and located so that they do not contaminate ground water away from the orebody. Uranium ISL uses the native groundwater in the orebody which is fortified with a complexing agent and in most cases an oxidant. It is then pumped through the underground orebody to recover the minerals in it by leaching. Once the pregnant solution is returned to the surface, the uranium is recovered in much the same way as in any other uranium plant (mill). In Australian ISL mines (Beverley and the soon to be opened Honeymoon Mine) the oxidant used is hydrogen peroxide and the complexing agent sulfuric acid. -

Oct. 14, 1958 J. M. CARTER ET AL 2,856,263 PROCESS for the RECOVERY and PURIFICATION of URANIUM DEPOSITS Original Filed April 21, 1944 ‘7 Sheets-Sheet 1
Oct. 14, 1958 J. M. CARTER ET AL 2,856,263 PROCESS FOR THE RECOVERY AND PURIFICATION OF URANIUM DEPOSITS Original Filed April 21, 1944 ‘7 Sheets-Sheet 1 IN VEN TORS James M Car/‘er Mar?n D. ?ame/7 BY ATTORNEY Oct. 14, 1958 J. M. CARTER ET AL 2,856,263 PROCESS FOR THE RECOVERY AND PURIFICATION OF URANIUM DEPOSITS Original Filed April 21, 1944 7 Sheets-Sheet 55 QQOUMM,vRMmG w.9386mmE IN VEN TORS James M. Car fer By Marfin 0 Home/7 W4 W ATTORNEY Oct. 14,1958 J. M. CARTER ET AL 2,856,263 PROCESS FOR THE RECOVERY AND PURIFICATION _ OF URANIUM DEPOSITS Origlnal Filed April 21, 1944 7 Sheets-Sheet 4 SCRUBBYING. AND WASHING PARTS OF CALUTRON ' WITH HOT WATER Lf- WASH WATER :FCONDENSATE ' \WATERANDMAKE UP snevmc SOL/D IMPUR/ TIES — WASH WA TER -—p>- CONDENSING To DISCARD I OXIDIZING ORSALVAGE SOLUTION U02” Cu?! Few’! Cr!!! Ni“ FILTERING‘ - '3 PREC/P/TATE 0* |——‘———/ F/LTRA TE _ U024!- TO DISCARD __ EVAPORATING gel”,++ OR SALVAGE PRECIPITATING Z/VH OH AND F'LTER'NG gFlLTRA TE TO FURTHER T REATMENT Fig. 4 I INVENTORS ‘James M Cor fer, BY Mar/7'0 D. Kama/7 ' @ M A TTORNEY Oct. 14,1958 J. M. CARTER ET AL . 2,356,263 PROCESS FOR THE RECOVERY AND PURIFICATION 0F URANIUM DEPOSITS Original Filed April 21, 1944 7 Sheets-Sheet s DISSOLVING URANIUM METAL DEPOSITED ON COPPER COLLECTOR IN COLD HNo3 (8N) CONDENSATE I AND MAKE UP SOLUTION HNO3 U02" ' Cu ** 7—>— CONDENSING ' HN03 . EVAPORATI NG CONCENTRA TED \ soburj'o/v _ F/LTRA TE C35 cu {NHJLF H PRECIPITATING ‘ -[—-~’—NH4OH A ND F ILTERING ' f/RECIP/ TA TES *Cu/‘OHZH4 :0 01 ORTO SALVAGEDISCARD *Trace T__>_ DISSOLVING HN 0 3 ' - SOLUTION U02 +4 *cuvf *Tme F/LTRA TE PRECIPITATING _l—_'_NH OH AND FILTERING 4 { PREClP/TATE NH U 0 TO DISCARD ( ‘)2 2 ’ OR SALVAGE , r---‘“—? TO FURTHER TREATMENT 5 I N V EN TORS' James M Car/‘er ' Mar/"in D. -

Reactor Centrum Nederland
RCN H-lfS REACTOR CENTRUM NEDERLAND RCX-186 INVESTIGATION'S ON' l'RAN>L CHLORIDE, ITS HYDRATES, AND BAL;I" SALTS by G. Prins RCN does not assume any liabili;;' with respect to the use of, or for damages resulting from the use of any information, apparatus, method or process disclosed in this document. is: >?:'': as o. t><?~ifi, Vr i"verr\ity \T.'J '.•'••:":• Petten, May 1973. St'MMARY 'ibis report describes the preparation and an inwst: .-at io:. physieo-cheniical properties ot uranyl .-hlorivie, its i.y.-.r .*.•. 01 its basic sales. The Methods lor Lhe synthesis ot l'i'.,i\,, l'U .i!L , .'t; ,i , .U'.vi i; li«vo beer. critically reviewed in Chapter Li and L:U: :::a:<> ru been careiulLy checked. In order to obtain more information on phase rv latienshiy :- uranyl chloride - water system, the system I'O - \iC\ - •• ,i been investigated (Chapter III;. Five solubility rogi.ns :. round with the corresponding solid phases ."i>,i:i ..JH.O, l\> ,•.i. : i .•: . 2UO.HCl.4H.vO, 4U0 .HCl ÖH,Ü, and L'O .2ri,0. One oi the throe basie salts, 4U0 .HCL.8H ,0, is tsetastable. This compound has no. heen described before. Since the basic salt ro,(OH)C1.2H,0 is congruently soluhle, it ran '.-.- prepared easily. Vibrational spectra of U0oJio, its hydrates, l'0,<oh)C1.2H,u, i'i',. Jt-,i>. and lour other uranyl compounds have been obtained (Chapt.r l\'). !:;.• interpretation of these spectra has heen restricted to a discussion ot the stretching frequencies of the uranyl group. -

ATSDR Toxicological Profile for Radon
TOXICOLOGICAL PROFILE FOR RADON U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry May 2012 RADON ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. RADON iii UPDATE STATEMENT A Toxicological Profile for Radon, Draft for Public Comment was released in September 2008. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Human Health Sciences (proposed)/ Environmental Toxicology Branch (proposed) 1600 Clifton Road NE Mailstop F-62 Atlanta, Georgia 30333 RADON iv This page is intentionally blank. RADON v FOREWORD This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the toxic substances each profile describes. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. -

Informs Commission That Remedial Action Has Been Completed At
o n n - - - - - - ~ ~ ~ ~ ~ ~ _ , I........................ i RELEASED TO THE PDR: i :f''% dashg - a, : faJ i ese : \...../ . .inus....... POLICY ISSUE 77 33 (NEGATIVE CONSENT) March 13, 1997 SECY-97-061 FOR: The Commissioners FROM: L. Joseph Callan Executive Director for Operations SUBJECT. REMOVAL OF TEXAS INSTRUMENTS. INC. FROM SITE DECOMMISSIONING MANAGEMENT PLAN PURPOSE: To inform the Commission that remedial action nas been completed at the Texas Instruments. Inc. (TI) site in Attleboro. Massachusetts. The staff plans to approve release of the site for unrestricted use terminate the current Nuclear Regulatory Commission license, and remove the site from the Site Decommissioning Management Plan (SDMP). SUMMARY: TI conducted uranium operations from 1952 to 1981. The licensee has now satisfactorily remediated the site. Based on the actions taken by the licensee, staff review of the surveys performed, and the results of the confirmatory survey, the staff plans to terminate the license before Massachusetts becomes an Agreement State on March 21, 1997. A representative of the Commonwealth of Massachusetts,- Department of Public Health - Radiation Control Program accompanied and assisted Region I staff during the final confirmatory survey. Massachusetts represertatives indicate they have no unresolved concerns about the NRC-regulated radiological material at the site and have confirmed in a letter to Region I that documentation provided by the licensee is sufficient to demonstrate compliance with Massachusetts' Contact: M. Roberts. RI NOTE: To BE MADE PUBLICLY AVAILABLE WHEN : (610) 337-5094 THE FINAL SRM IS MADE AVAILABLE ~ d ' _7m -)Sl*t h f W0 2 c ?OW 0- /0S mainitto!w X /9 dtS MzzzA 'MHMzzzzzzzA . -

ORGANOMETAT,T,TC CHEMISTRY of URANIUM a Thesis Submitted By
ORGANOMETAT,T,TC CHEMISTRY OF URANIUM A thesis submitted by R1TN R. SIGURDSON, B.Sc. for the DEGREE of DOCTOR of PHILOSOPHY of the UNIVERSITY of LONDON Royal College of Science Imperial College of Science and Technology London, SW7 ?AY August 1976 TO MY PARENTS 3 ACKNOWLEDGEMENTS I would like to express my gratitude to Professor Geoffrey Wilkinson, F.R.S. for his guidance and enthusiastic support throughout the course of this work. Many thanks are also extended to Drs. Dick Andersen, Ernesto Carmona-Guzman and David Cole-Hamilton for their suggestionS, encouragement and advice, and to Dr. Kostas Mertis for his patient help during the first months. I am indebted to the Canadian Research Council of Canada for financial support during the past three years. 4 CONTENTS ABSTRACT 6 INTRODUCTION I. The Chemistry of Uranium(IV) 8 .II. The Chemistry of Uranium(V) 15 III. The Chemistry of Uranium(VI) 16 CHAPTER I. DILITHIUMHEXAALKYLURANATE(IV) COMPLEXES I. Introduction 19 II. Results and Discussion 27 III. Experimental 35 CHAPTER II. TRILITHIUMOCTAALKYLURANATE(V) COMPLEXES I. Introduction 54 II. Results and Discussion 55 III. Experimental 60 CHAPTER III. ADDITION COMPOUNDS OF URANIUM(VI) HEXAISO-PROPDXIDE WITH LITHIUM, MAGNESIUM AND ALUMINIUM ALKYLS I. Introduction 70 II. Results and Discussion 71 III. Experimental 77 CHAPTER IV. ORGANOMETALLIC CHEMISTRY OF ADAMANTANE I. Introduction 84 II. Results and Discussion 85 III. Experimental 87 REFERENCES 92 5 ABBREVIATIONS Me - methyl Et - ethyl Prn- normal-propyl Pri- iso-propyl Bun- normal-butyl But- iso-butyl But- tertiary-butyl Ph - phenyl CP cyclopentadienyl DME - dimethoxyethane tmed - N,N,NI,N'-tetramethylethylenediamine pmdt - N,N,Nt,N",N"-pentamethyldiethylenetriamine g.l.c. -

Radon-A Physician's Guide: the Health
Radon-A Physician's Guide: The Health Threat With A Simple Solution U.S. Environmental Protection Agency Office of Air and Radiation Indoor Environments Division (6609J) 1. Executive Summary 10. 2. What is Radon? 3. Characteristics and Source of Radon 4. The Health Risk o How does Radon Induce cancer? o What is the Evidence? o Is Occupational Exposure to Radon Comparable to Residential Exposure? 5. What About Smoking and Radon Exposure? The Solution o Why Should Every Home be Tested? o How Do You Obtain a Reliable Test Result? o Radon Testing Methods o Radon Test Devices o How to Test o Interpreting Radon Test Results o Basis for the 4 pCi/L Radon "Action Level" o Radon Reduction Methods 6. Other Indoor Air Pollutants o Environmental Tobacco Smoke (ETS) o Biological Air Pollutants o Volatile Organic Compounds (VOCs) o Other Combustion Products 7. Most Commonly Asked Questions about Radon 8. State and Regional Radon Contacts 9. Additional Information Sources o Radon Publications o Radon Hotlines Introduction Lung cancer's very high associated mortality rate is even more tragic because a significant portion of lung cancer is preventable. While smoking remains the number one cause of lung cancer, radon presents a significant second risk factor. That is why, in addition to encouraging patients to stop smoking, it is important for physicians to inquire about and encourage patients to test for radon levels in their homes. One way to do this is for physicians to join those health care professionals and organizations who have begun to include questions about the radon level in patients' homes on standardized patient history forms. -

Sources, Effects and Risks of Ionizing Radiation
SOURCES, EFFECTS AND RISKS OF IONIZING RADIATION United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2016 Report to the General Assembly, with Scientific Annexes UNITED NATIONS New York, 2017 NOTE The report of the Committee without its annexes appears as Official Records of the General Assembly, Seventy-first Session, Supplement No. 46 and corrigendum (A/71/46 and Corr.1). The report reproduced here includes the corrections of the corrigendum. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The country names used in this document are, in most cases, those that were in use at the time the data were collected or the text prepared. In other cases, however, the names have been updated, where this was possible and appropriate, to reflect political changes. UNITED NATIONS PUBLICATION Sales No. E.17.IX.1 ISBN: 978-92-1-142316-7 eISBN: 978-92-1-060002-6 © United Nations, January 2017. All rights reserved, worldwide. This publication has not been formally edited. Information on uniform resource locators and links to Internet sites contained in the present publication are provided for the convenience of the reader and are correct at the time of issue. The United Nations takes no responsibility for the continued accuracy of that information or for the content of any external website. -

Final Program
1 General Information SCOPE OF THE CONFERENCE The 11th Joint MMM/Intermag Conference is sponsored jointly by the American Institute of Physics (PCI) and the Magnetics Society of the IEEE, in cooperation with The American Physical Society. Members of the inter- national scientific and engineering communities interested in recent devel- opments in fundamental and applied magnetism are invited to attend the Conference and contribute to its technical sessions. Sessions will include invited and contributed papers, oral and poster presentations and invited symposia. This Conference provides an outstanding opportunity for partici- pants to meet their colleagues and discuss new, advanced and controversial developments. WASHINGTON, DC The 2010 Joint Conference will be held in the nation’s capital. The city wel- comes 15 million visitors each year of which 1.2 million are international. There are more than 100 restaurants located in the downtown area alone, and the city has been called “one of the most exciting restaurant cities on the East Coast” by Travel & Leisure. To obtain an in-depth and current view of what to see and do while you are here, visit the official website at: http://www.washington.org. Here you will find information on how to trav- el to the Marriott Washington Wardman Park from any of the three local air- ports; you can check on the weather in mid-January, and can request a free Visitors Guide prior to making your trip. VISA REQUIREMENTS The US has updated its visa policies to increase security, so it may take you 3-6 months to apply for and receive your visa. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials.