Phylogeny and Taxonomy of the Neotropical Thepytus (Lepidoptera: Lycaenidae: Theclinae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

North Andean Origin and Diversification of the Largest Ithomiine Butterfly Genus
North Andean origin and diversification of the largest ithomiine butterfly genus The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Lisa De-Silva, D., L. L. Mota, N. Chazot, R. Mallarino, K. L. Silva- Brandão, L. M. G. Piñerez, A. V. Freitas, et al. 2017. “North Andean origin and diversification of the largest ithomiine butterfly genus.” Scientific Reports 7 (1): 45966. doi:10.1038/srep45966. http:// dx.doi.org/10.1038/srep45966. Published Version doi:10.1038/srep45966 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:32630680 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA www.nature.com/scientificreports OPEN North Andean origin and diversification of the largest ithomiine butterfly genus Received: 31 October 2016 Donna Lisa De-Silva1, Luísa L. Mota2, Nicolas Chazot1,3, Ricardo Mallarino4, Karina L. Silva- Accepted: 22 February 2017 Brandão5, Luz Miryam Gómez Piñerez6,7, André V.L. Freitas2, Gerardo Lamas8, Published: 07 April 2017 Mathieu Joron9, James Mallet4, Carlos E. Giraldo6,10, Sandra Uribe6, Tiina Särkinen11, Sandra Knapp12, Chris D. Jiggins13, Keith R. Willmott14 & Marianne Elias1 The Neotropics harbour the most diverse flora and fauna on Earth. The Andes are a major centre of diversification and source of diversity for adjacent areas in plants and vertebrates, but studies on insects remain scarce, even though they constitute the largest fraction of terrestrial biodiversity. -

Lepidopterofauna (Papilionoidea E Hesperioidea) Do Parque Estadual Do Chandless E Arredores, Acre, Brasil
Biota Neotrop., vol. 10, no. 4 Lepidopterofauna (Papilionoidea e Hesperioidea) do Parque Estadual do Chandless e arredores, Acre, Brasil Olaf Hermann Hendrik Mielke1,2, Eduardo Carneiro¹ & Mirna Martins Casagrande¹ ¹Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná – UFPR, CP 19020, CEP 81531-980, Curitiba, PR, Brasil 2Autor para correspondência: Olaf Hermann Hendrik Mielke, e-mail: [email protected] MIELKE, O.H.H., CARNEIRO, E. & CASAGRANDE, M.M. Lepidopterofauna (Papilionoidea e Hesperioidea) of the Parque Estadual do Chandless and surroundings, Acre, Brazil. Biota Neotrop. 10(4): http://www. biotaneotropica.org.br/v10n4/en/abstract?inventory+bn03210042010. Abstract: Given the absence of Lepidoptera inventories in the State of Acre and its scarcity in the Brazilian Amazon forest, this study aimed to list the species of Hesperioidea and Papilionoidea present in the Parque Estadual do Chandless and surroundings. The access to the region is complicated and it has no infrastructure for scientific research. During 14 days, the butterflies were collected with entomological nets, traps and Ahrenholz’s technique in different environments in the park and its surroundings. A total of 482 species were identified, none of them present in red lists of endangered species. It is expected a significantly greater number of species after the addition of new collections in other seasons, as the Jacknife 1 estimate does not reach its asymptote, or as compared to inventories in nearby areas that list nearly 1700 species after a greater sampling effort. Keywords: amazonian forest, butterflies, inventory, protected area. MIELKE, O.H.H., CARNEIRO, E. & CASAGRANDE, M.M. Lepidopterofauna (Papilionoidea e Hesperioidea) do Parque Estadual do Chandless e arredores, Acre, Brasil. -

Acta Zool. Hung. 53 (Suppl
Acta Zoologica Academiae Scientiarum Hungaricae 53 (Suppl. 1), pp. 211–224, 2007 THE DESCRIPTION OF THERITAS GOZMANYI FROM THE ANDES AND ITS SPECTROSCOPIC CHARACTERIZATION WITH SOME NOTES ON THE GENUS (LEPIDOPTERA: LYCAENIDAE: EUMAEINI) BÁLINT, ZS.1, WOJTUSIAK, J.2, KERTÉSZ, K.3 and BIRÓ, L. P.3 1Department of Zoology, Hungarian Natural History Museum H-1088 Budapest, Baross u. 13, Hungary; E-mail: [email protected] 2Zoological Museum, Jagiellonian University, 30–060 Kraków, Ingardena 6, Poland 3Department of Nanotechnology, Research Institute for Technical Physics and Material Science H-1525 Budapest, P.O. Box 49, Hungary A key for separating sister genera Arcas SWAINSON, 1832 and Theritas HÜBNER, 1818, plus eight nominal species placed in Theritas is given. Three species groups within the latter genus are distinguished. A new species, Theritas gozmanyi BÁLINT et WOJTUSIAK, sp. n. is described form Ecuador. The presence of a discal scent pad on the fore wing dorsal surface and spectral characteristics of the light reflected from the central part of the discal cell were used as charac- ters for discrimination of the new species. Key words: androconial clusters, spectroscopy, structural colours, Theritas species-groups INTRODUCTION The generic name Theritas was established by monotypy for the new species Theritas mavors by HÜBNER (1818). The genus-group name was not in general use until the revision of D’ABRERA (1995), who placed 23 species-group taxa in Theritas on the basis of the character “the pennent-like tail which projects out- wards, and an approximate right angle from, and as a part of, a squared-off projec- tion of the tapered h. -

INSECTA MUNDIA Journal of World Insect Systematics
INSECTA MUNDI A Journal of World Insect Systematics 0506 Annotated checklist and biogeographic composition of the Lycaenidae (Lepidoptera) of Trinidad, West Indies Matthew J.W. Cock CABI, Bakeham Lane Egham, Surrey, TW20 9TY United Kingdom Robert K. Robbins Smithsonian Institution PO Box 37012, NHB Stop 105 (E-514) Washington, DC 20013-7012 USA Date of Issue: October 21, 2016 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Matthew J.W. Cock and Robert K. Robbins Annotated checklist and biogeographic composition of the Lycaenidae (Lepidoptera) of Trinidad, West Indies Insecta Mundi 0506: 1–33 ZooBank Registered: urn:lsid:zoobank.org:pub:37A7668A-0D83-4DB0-BD28-C36302F18398 Published in 2016 by Center for Systematic Entomology, Inc. P. O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non-marine arthropod. Topics considered for publication include systematics, taxonomy, nomenclature, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medical entomology, pest control research, etc.), and no longer publishes book reviews or editorials. Insecta Mundi publishes original research or discoveries in an inexpensive and timely manner, distributing them free via open access on the internet on the date of publication. Insecta Mundi is referenced or abstracted by several sources including the Zoological Record, CAB Ab- stracts, etc. Insecta Mundi is published irregularly throughout the year, with completed manuscripts assigned an individual number. Manuscripts must be peer reviewed prior to submission, after which they are reviewed by the editorial board to ensure quality. -

Diversity, Ecology and Herbivory of Hairstreak Butterflies (Theclinae) Associated with the Velvet Tree, Miconia Calvescens in Costa Rica Author(S): F
Diversity, Ecology and Herbivory of Hairstreak Butterflies (Theclinae) Associated with the Velvet Tree, Miconia calvescens in Costa Rica Author(s): F. R. Badenes-Pérez, M. A. Alfaro-Alpízar and M. T. Johnson Source: Journal of Insect Science, 10(209):1-9. 2010. Published By: Entomological Society of America DOI: http://dx.doi.org/10.1673/031.010.20901 URL: http://www.bioone.org/doi/full/10.1673/031.010.20901 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Insect Science: Vol. 10 | Article 209 Badenes-Pérez et al. Diversity, ecology and herbivory of hairstreak butterflies (Theclinae) associated with the velvet tree, Miconia calvescens in Costa -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Proceedings of the Arkansas Academy of Science - Volume 29 1975 Academy Editors
Journal of the Arkansas Academy of Science Volume 29 Article 1 1975 Proceedings of the Arkansas Academy of Science - Volume 29 1975 Academy Editors Follow this and additional works at: https://scholarworks.uark.edu/jaas Recommended Citation Editors, Academy (1975) "Proceedings of the Arkansas Academy of Science - Volume 29 1975," Journal of the Arkansas Academy of Science: Vol. 29 , Article 1. Available at: https://scholarworks.uark.edu/jaas/vol29/iss1/1 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Entire Issue is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. \ ProceedingsJournal of theof Arkansasthe Academy of Science, Vol. 29 [1975], Art. 1 CODEN: AKASO ARKANSAS ACADEMYOF SCIENCE VOLUMEXXVIV 1975 ARKANSAS ACADEMYOF SCIENCE UNIVERSITY OF ARKANSASBOX 2407 FAYETTEVILLE,ARKANSAS72701 Published by Arkansas AcademySPECIAL of Science,4th CLASS 1975 1 BOOK RATE Journal of the Arkansas Academy of Science, Vol. 29 [1975], Art. 1 Arkansas Academy of Science, University of Arkansas, Box 2407 Fayetteville, Arkansas 72701 INSTITUTIONALMEMBERS -
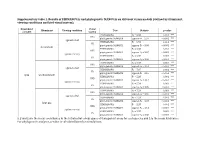
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

Family LYCAENIDAE: 268 Species GOSSAMERWINGS
Family LYCAENIDAE: 268 species GOSSAMERWINGS Subfamily Miletinae: 1 (hypothetical) species Harvesters Feniseca tarquinius tarquinius Harvester Hypothetical, should occur in N Tamaulipas, but currently unknown from Mexico Subfamily Lycaeninae: 6 species Coppers Iophanus pyrrhias Guatemalan Copper Lycaena arota arota Tailed Copper Lycaena xanthoides xanthoides Great Copper Lycaena gorgon gorgon Gorgon Copper Lycaena helloides Purplish Copper Lycaena hermes Hermes Copper Subfamily Theclinae: 236 species Hairstreaks Tribe Theclini: 3 species Hairstreaks Hypaurotis crysalus crysalus Colorado Hairstreak Habrodais grunus grunus Golden Hairstreak verification required for Baja California Norte Habrodais poodiae Baja Hairstreak Tribe Eumaeini: 233 Hairstreaks Eumaeus childrenae Great Cycadian (= debora) Eumaeus toxea Mexican Cycadian Theorema eumenia Pale-tipped Cycadian Paiwarria antinous Felders' Hairstreak Paiwarria umbratus Thick-tailed Hairstreak Mithras sp. undescribed Pale-patched Hairstreak nr. orobia Brangas neora Common Brangas Brangas coccineifrons Black-veined Brangas Brangas carthaea Green-spotted Brangas Brangas getus Bright Brangas Thaeides theia Brown-barred Hairstreak Enos thara Thara Hairstreak Enos falerina Falerina Hairstreak Evenus regalis Regal Hairstreak Evenus coronata Crowned Hairstreak Evenus batesii Bates’ Hairstreak Atlides halesus corcorani Great Blue Hairstreak Atlides gaumeri White-tipped Hairstreak Atlides polybe Black-veined Hairstreak Atlides inachus Spying Hairstreak Atlides carpasia Jeweled Hairstreak Atlides -

BUTTERFLIES in Thewest Indies of the Caribbean
PO Box 9021, Wilmington, DE 19809, USA E-mail: [email protected]@focusonnature.com Phone: Toll-free in USA 1-888-721-3555 oror 302/529-1876302/529-1876 BUTTERFLIES and MOTHS in the West Indies of the Caribbean in Antigua and Barbuda the Bahamas Barbados the Cayman Islands Cuba Dominica the Dominican Republic Guadeloupe Jamaica Montserrat Puerto Rico Saint Lucia Saint Vincent the Virgin Islands and the ABC islands of Aruba, Bonaire, and Curacao Butterflies in the Caribbean exclusively in Trinidad & Tobago are not in this list. Focus On Nature Tours in the Caribbean have been in: January, February, March, April, May, July, and December. Upper right photo: a HISPANIOLAN KING, Anetia jaegeri, photographed during the FONT tour in the Dominican Republic in February 2012. The genus is nearly entirely in West Indian islands, the species is nearly restricted to Hispaniola. This list of Butterflies of the West Indies compiled by Armas Hill Among the butterfly groupings in this list, links to: Swallowtails: family PAPILIONIDAE with the genera: Battus, Papilio, Parides Whites, Yellows, Sulphurs: family PIERIDAE Mimic-whites: subfamily DISMORPHIINAE with the genus: Dismorphia Subfamily PIERINAE withwith thethe genera:genera: Ascia,Ascia, Ganyra,Ganyra, Glutophrissa,Glutophrissa, MeleteMelete Subfamily COLIADINAE with the genera: Abaeis, Anteos, Aphrissa, Eurema, Kricogonia, Nathalis, Phoebis, Pyrisitia, Zerene Gossamer Wings: family LYCAENIDAE Hairstreaks: subfamily THECLINAE with the genera: Allosmaitia, Calycopis, Chlorostrymon, Cyanophrys, -

Describing Species
DESCRIBING SPECIES Practical Taxonomic Procedure for Biologists Judith E. Winston COLUMBIA UNIVERSITY PRESS NEW YORK Columbia University Press Publishers Since 1893 New York Chichester, West Sussex Copyright © 1999 Columbia University Press All rights reserved Library of Congress Cataloging-in-Publication Data © Winston, Judith E. Describing species : practical taxonomic procedure for biologists / Judith E. Winston, p. cm. Includes bibliographical references and index. ISBN 0-231-06824-7 (alk. paper)—0-231-06825-5 (pbk.: alk. paper) 1. Biology—Classification. 2. Species. I. Title. QH83.W57 1999 570'.1'2—dc21 99-14019 Casebound editions of Columbia University Press books are printed on permanent and durable acid-free paper. Printed in the United States of America c 10 98765432 p 10 98765432 The Far Side by Gary Larson "I'm one of those species they describe as 'awkward on land." Gary Larson cartoon celebrates species description, an important and still unfinished aspect of taxonomy. THE FAR SIDE © 1988 FARWORKS, INC. Used by permission. All rights reserved. Universal Press Syndicate DESCRIBING SPECIES For my daughter, Eliza, who has grown up (andput up) with this book Contents List of Illustrations xiii List of Tables xvii Preface xix Part One: Introduction 1 CHAPTER 1. INTRODUCTION 3 Describing the Living World 3 Why Is Species Description Necessary? 4 How New Species Are Described 8 Scope and Organization of This Book 12 The Pleasures of Systematics 14 Sources CHAPTER 2. BIOLOGICAL NOMENCLATURE 19 Humans as Taxonomists 19 Biological Nomenclature 21 Folk Taxonomy 23 Binomial Nomenclature 25 Development of Codes of Nomenclature 26 The Current Codes of Nomenclature 50 Future of the Codes 36 Sources 39 Part Two: Recognizing Species 41 CHAPTER 3.