Capturing Participant Data for Mucosal Sample Interpretation: a Guide for HIV Investigators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for High-Grade Anal Intraepithelial Neoplasia
Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for High-Grade Anal Intraepithelial Neoplasia Using The BARRX™ Anorectal Wand (Clinical Protocol 01-2017) AUGUST 31, 2017 Page 1 of 40 Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia PROTOCOL SIGNATURE PAGE I, , Principal Investigator at site , agree to conduct and follow this protocol: PROTOCOL 01-2017: A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for Anal Intraepithelial Neoplasia using the Barrx™ Anorectal Wand as written according to FDA guidelines. I understand that no deviations from the above protocol may be made without written permission from the Protocol Chair(s). _________________________________ _____________________ Signature Date (mm/dd/yyyy) Page 2 of 40 Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia P ROT O COL S U M M ARY Title A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for Anal Intraepithelial Neoplasia using the Barrx™ Anorectal Wand Design Multi-center prospective trial involving up to 70 subjects Subject Population HIV-positive and HIV-negative subjects with intra-anal intraepithelial neoplasia (AIN) containing at least one high- grade squamous intraepithelial lesions (HSIL) involving the squamocolumnar junction (SCJ). Objective Assess the safety, and efficacy of circumferential radiofrequency ablation (RFA) to the anal canal using the FDA cleared Barrx™ Anorectal Wand to eradicate anal -

IAVI & Partners' Presentations at HIV R4P 2016
Main Program Highlights Protection from Rectal SHIV Infection Induced by Mucosal Vaccination with a Replication-competent VSV-HIV Chimera Delivering Env Trimers (Symposium Presentation) Date: Tuesday, 18 October Time: 15:52-16:14 Venue: Sheraton Grand Chicago, Chicago Ballroom VI-VII Chairs: Bonnie Mathieson, Office of AIDS Research; Etienne Karita, Projet San Francisco Presenter: Chris Parks, IAVI AAV and Other Novel Antibody Delivery Platforms (Symposium Presentation) Date: Wednesday, 19 October Time: 16:14-16:36 Venue: Sheraton Grand Chicago, Sheraton Ballroom IV-V Chairs: Magdalena E Sobieszczyk, College of Physicians and Surgeons, Columbia University; Glenda Gray, South African Medical Research Council Presenter: Frances Priddy, IAVI When We Got It Right and When We Got It Wrong (Round Table Presentation) Date: Thursday, 20 October Time: 8:30-8:40 Venue: Sheraton Grand Chicago, Chicago Ballroom VIII-X Chair: Mitchell Warren, AVAC Presenter: Maggie Keane, IAVI For full program details go to: http://www.iavi.org/events/599-hivr4p-2016 Satellite Symposium HIV Vaccine Design and Development Partnerships in Africa: Showcasing African-led Basic HIV Prevention Science Research Monday 17 October 2016, 8:30 - 12:30 Sheraton Grand Chicago, Chicago Ballroom IX Refreshments provided This session will bring together African and international scientists, policy makers, advocates and institutions involved in HIV prevention research. They will take a look at current design and development partnership models to identify success factors, gaps and opportunities for enhanced collaborations, as well as young scientists. Presenters and participants will be invited to share their ideas of the next priorities for African HIV prevention research. These will be discussed in a panel discussion on the way forward. -
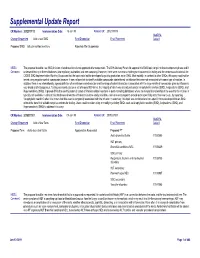
Detail Report
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

Acceptability of Potential Rectal Microbicide Delivery Systems for HIV Prevention: a Randomized Crossover Trial
AIDS Behav DOI 10.1007/s10461-012-0358-z ORIGINAL PAPER Acceptability of Potential Rectal Microbicide Delivery Systems for HIV Prevention: A Randomized Crossover Trial Heather A. Pines • Pamina M. Gorbach • Robert E. Weiss • Kristen Hess • Ryan Murphy • Terry Saunders • Joelle Brown • Peter A. Anton • Ross D. Cranston Ó Springer Science+Business Media New York 2012 Abstract We assessed the acceptability of three of over- acceptability and should be considered early in RM devel- the-counter products representative of potential rectal opment to enhance potential use. microbicide (RM) delivery systems. From 2009 to 2010, 117 HIV-uninfected males (79 %) and females (21 %) who Keywords Anorectal products Á Receptive anal engage in receptive anal intercourse participated in a 6-week intercourse Á HIV prevention randomized crossover acceptability trial. Participants received each of three products (enema, lubricant-filled applicator, suppository) every 2 weeks in a randomized Introduction sequence. CASI and T-ACASI scales assessed product acceptability via Likert responses. Factor analysis was used to Rectal microbicides (RM) can be formulated as gels, creams, identify underlying factors measured by each scale. Random or enemas for application inside the rectum to prevent HIV effects models were fit to examine age and gender effects on infection during anal intercourse (AI). Among 24,787 men product acceptability. Three underlying factors were identi- who have sex with men (MSM) who completed an online fied: Satisfaction with Product Use, Sexual Pleasure,and survey in the United States in 2010, 36 and 34 % reported Ease of Product Use. For acceptability, the applicator ranked having receptive anal intercourse (RAI) and insertive anal highest; however, differences between product acceptability intercourse (IAI) at last sex, respectively [1]. -

Associated Lymphoid Tissue in Chickens and Turkeys Andrew Stephen Fix Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1990 The trs ucture and function of conjunctiva- associated lymphoid tissue in chickens and turkeys Andrew Stephen Fix Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Animal Sciences Commons, and the Veterinary Medicine Commons Recommended Citation Fix, Andrew Stephen, "The trs ucture and function of conjunctiva-associated lymphoid tissue in chickens and turkeys " (1990). Retrospective Theses and Dissertations. 9496. https://lib.dr.iastate.edu/rtd/9496 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. Hie quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

The Evolution of Nasal Immune Systems in Vertebrates
Molecular Immunology 69 (2016) 131–138 Contents lists available at ScienceDirect Molecular Immunology j ournal homepage: www.elsevier.com/locate/molimm The evolution of nasal immune systems in vertebrates ∗ Ali Sepahi, Irene Salinas Center for Evolutionary and Theoretical Immunology, Department of Biology, University of New Mexico, Albuquerque, NM, USA a r t i c l e i n f o a b s t r a c t Article history: The olfactory organs of vertebrates are not only extraordinary chemosensory organs but also a powerful Received 15 July 2015 defense system against infection. Nasopharynx-associated lymphoid tissue (NALT) has been traditionally Received in revised form 5 September 2015 considered as the first line of defense against inhaled antigens in birds and mammals. Novel work in Accepted 6 September 2015 early vertebrates such as teleost fish has expanded our view of nasal immune systems, now recognized Available online 19 September 2015 to fight both water-borne and air-borne pathogens reaching the olfactory epithelium. Like other mucosa- associated lymphoid tissues (MALT), NALT of birds and mammals is composed of organized lymphoid Keywords: tissue (O-NALT) (i.e., tonsils) as well as a diffuse network of immune cells, known as diffuse NALT (D- Evolution NALT). In teleosts, only D-NALT is present and shares most of the canonical features of other teleost Nasal immunity NALT MALT. This review focuses on the evolution of NALT in vertebrates with an emphasis on the most recent Mucosal immunity findings in teleosts and lungfish. Whereas teleost are currently the most ancient group where NALT has Vertebrates been found, lungfish appear to be the earliest group to have evolved primitive O-NALT structures. -

Infectious Gastroenteritis Generalised Anxiety Disorder HIV Smoking Associated Cancers
BEST PRACTICE 25 DECEMBER 2009 Infectious gastroenteritis Generalised anxiety disorder HIV bpac nz Smoking associated cancers better medicin e Editorial Team Tony Fraser Professor Murray Tilyard We would like to acknowledge the following people for Clinical Advisory Group their guidance and expertise in developing this edition: Michele Cray Dr Shaun Costello, Dunedin Serena Curtis-Lemuelu Dr Edward Coughlan, Christchurch Dr Rosemary Ikram Professor Tony Dowell, Wellington Dr Cam Kyle Dr Rosemary Ikram, Christchurch Dr Chris Leathart Mr William Pearce, Christchurch Dr Lynn McBain Dr Alan Pithie, Christchurch Adam McRae Dr Gabrielle Ruben, Wellington Janet Maloney-Moni Assoc. Professor Mark Thomas, Auckland Dr Peter Moodie Dr Robyn Toomath, Wellington Associate Professor Jim Reid Dr Neil Whittaker, GP Reviewer, Nelson Associate Professor David Reith Assoc. Professor Michael Williams, Dunedin Professor Murray Tilyard Programme Development Team Rachael Clarke Peter Ellison Best Practice Journal (BPJ) Rebecca Harris Julie Knight ISSN 1177-5645 Noni Richards BPJ, Issue 25, December 2009 Dr Tom Swire Dr AnneMarie Tangney nz Dr Sharyn Willis BPJ is published and owned by bpac Ltd Dave Woods Level 8, 10 George Street, Dunedin, New Zealand. Report Development Team Bpacnz Ltd is an independent organisation that promotes health Justine Broadley care interventions which meet patients’ needs and are evidence Todd Gillies based, cost effective and suitable for the New Zealand context. Lana Johnson We develop and distribute evidence based resources which describe, facilitate and help overcome the barriers to best Web practice. Gordon Smith Bpacnz Ltd is currently funded through contracts with PHARMAC Design and DHBNZ. Michael Crawford Bpacnz Ltd has five shareholders: Procare Health, South Link Management and Administration Health, IPAC, the University of Otago and Pegasus Health. -

Pathology of Anal Cancer
Pathology of Anal Cancer a b Paulo M. Hoff, MD, PhD , Renata Coudry, MD, PhD , a, Camila Motta Venchiarutti Moniz, MD * KEYWORDS Anal cancer Anal squamous intraepithelial neoplasia Squamous cell carcinoma Human papilloma virus (HPV) Molecular KEY POINTS Anal cancer is an uncommon tumor, squamous cell carcinoma (SCC) being the most frequent histology corresponding to 80% of all cases. Human papilloma virus (HPV) infection plays a key role in anal cancer development, en- coding at least three oncoproteins with stimulatory properties. SCC expresses CK5/6, CK 13/19, and p63. P16 is a surrogate marker for the presence of HPV genome in tumor cells. INTRODUCTION Anal cancer accounts for approximately 2.4% of gastrointestinal malignancies.1 Although anal cancer is a rare tumor, its frequency is increasing, especially in high- risk groups.2 Tumors in this location are generally classified as anal canal or anal margin. Squamous cell carcinoma (SCC) is the predominant type of tumor and shares many features with cervical cancer. Oncogenic human papilloma virus (HPV) infection plays a major role in both tumors.3 HIV infection is associated with a higher frequency of HPV-associated premalignant lesions and invasive tumors.4 Normal Anatomy of the Anus The anal canal is the terminal part of the large intestine and is slightly longer in male than in female patients. It measures approximately 4 cm and extends from the rectal ampulla (pelvic floor level) to the anal verge, which is defined as the outer open- ing of the gastrointestinal tract. The anal verge is at the level of the squamous- mucocutaneous junction with the perianal skin.5,6 The authors have nothing to disclose. -

HPV, Anal Dysplasia and Anal Cancer
HPV, anal dysplasia FACT and anal cancer SHEET Published 2016 Summary Anal cancer typically develops over a period of years, beginning with a precancerous condition called anal dysplasia. CONTACT US Anal dysplasia occurs when clusters of abnormal cells form by telephone lesions in the mucosa lining of the anal canal (between the 1-800-263-1638 anus and the rectum). The lesions typically form inside the anal 416-203-7122 canal or just outside the anal opening. by fax 416-203-8284 Although there are over 100 different types of the human papillomavirus (HPV), anal dysplasia is usually caused by certain by e-mail strains of HPV which can be transmitted sexually. HPV can shut [email protected] off the proteins that help prevent dysplasia and cancer cells from developing, therefore leading to HPV-associated diseases by mail such as anal dysplasia. 555 Richmond Street West Suite 505, Box 1104 It is difficult to screen for anal dysplasia since the lesions are Toronto ON M5V 3B1 not detectable by routine examinations. As a result, anal dysplasia is often not detected until it has developed into anal cancer, which can be difficult to treat depending on the severity. Specific screening tests can detect dysplasia or precancerous changes. If these precancers are treated, anal cancer may be prevented. Anal cancer is usually treated with radiation and chemotherapy or with surgery. Although anal dysplasia may be treated successfully, individuals with HIV are at increased risk of it recurring and may need to be monitored closely by a trained physician. Consistent condom use reduces, but does not eliminate, the risk of transmitting HPV. -

Mucosal Immunity
Mucosal Immunity Lloyd Mayer, MD ABSTRACT. Food allergy is the manifestation of an antibody, secretory immunoglobulin A (sIgA), which abnormal immune response to antigen delivered by the is highly suited for the hostile environment of the gut oral route. Normal mucosal immune responses are gen- (Fig 1). All of these in concert eventuate in the im- erally associated with suppression of immunity. A nor- munosuppressed tone of the gastrointestinal (GI) mal mucosal immune response relies heavily on a num- tract. Defects in any individual component may pre- ber of factors: strong physical barriers, luminal digestion dispose to intestinal inflammation or food allergy. of potential antigens, selective antigen sampling sites, and unique T-cell subpopulations that effect suppres- sion. In the newborn, several of these pathways are not MUCOSAL BARRIER matured, allowing for sensitization rather than suppres- The mucosal barrier is a complex structure com- sion. With age, the mucosa associated lymphoid tissue posed of both cellular and noncellular components.5 matures, and in most individuals this allows for genera- Probably the most significant barrier to antigen entry tion of the normal suppressed tone of the mucosa asso- into the mucosa-associated lymphoid tissue (MALT) ciated lymphoid tissue. As a consequence, food allergies are largely outgrown. This article deals with the normal is the presence of enzymes starting in the mouth and facets of mucosal immune responses and postulates how extending down to the stomach, small bowel, and the different processes may be defective in food-allergic colon. Proteolytic enzymes in the stomach (pepsin, patients. Pediatrics 2003;111:1595–1600; gastrointestinal papain) and small bowel (trypsin, chymotrypsin, allergy, food allergy, food hypersensitivity, oral tolerance, pancreatic proteases) perform a function that they mucosal immunology. -

The Mediating Role of Condom Use and Rectal Bleeding in a National Onli
Epidemiology Sex Transm Infect: first published as 10.1136/sextrans-2019-054415 on 5 May 2020. Downloaded from Original research Association between rectal douching and HIV acquisition: the mediating role of condom use and rectal bleeding in a national online sample of Chinese men who have sex with men Tianyi Lu,1 Xiang Mao,1 Erlei Peng,1 Yangyang Gao,1 Zhenxing Chu,1 Willa Dong,2 Wenran Zhang,1 Yong- Jun Jiang,1 Junjie Xu 1 1NHC Key Laboratory of AIDS ABSTRact challenge imposed by the HIV epidemic among Immunology (China Medical Objectives Previous studies have demonstrated that certain populations, particularly MSM. National University), National Clinical Research Center for Laboratory rectal douching (RD) is associated with HIV acquisition surveillance data have indicated that the preva- Medicine, The First Affiliated among men who have sex with men (MSM). However, lence of HIV among MSM increased from 0.9% in Hospital of China Medical the precise mechanism underlying the association 2003 to 8% in 2015.3 4 Given the disproportion- University, Shenyang, China 2 between RD and HIV remains unclear. ately high burden of HIV infection among MSM, Department of Health Behavior, Methods We recruited participants over WeChat from it is essential to understand the sexual behaviours Gillings School of Global Public Health, The University of North October 2017 to October 2018. Respondents received and preferences of this key population in order to Carolina at Chapel Hill, Chapel mailed HIV self-testing kits, uploaded images of HIV develop a novel and effective strategy to prevent Hill, North Carolina, USA self-test results and completed an online electronic HIV infection. -

Mucosal Immunity Learning Goal
Host Defense 2013 Mucosal Immunity April 16, 2013 Katherine L.Knight, Ph.D. MUCOSAL IMMUNITY LEARNING GOAL You will be able to describe the mucosal immune system. OBJECTIVES To attain the goal for this lecture you will be able to: Describe the components of the mucosal immune system. Draw the structure of secretory IgA. Explain the mechanism of IgA transport across mucosal surfaces. Explain how a response to antigen is generated in the mucosal system. Identify the differences in tolerogenic versus immunogenic responses to mucosal antigen administration. Delineate the functions of the mucosal immune system, including M cells. Describe how the mucosal immune system might be used for immunization. Describe the characteristics of selective IgA deficiency. Describe how intestinal commensal bacteria interact with the host to promote a healthy environment READING ASSIGNMENT Janeway’s Immunobiology 8th Edition (2012), Chapter 12 and Article “Mucosal Immunity and Vaccines” by Robyn Seipp attached at end of lecture notes. LECTURER Katherine L. Knight, Ph.D. Page 1 Host Defense 2013 Mucosal Immunity April 16, 2013 Katherine L.Knight, Ph.D. CONTENT SUMMARY I. INTRODUCTION TO MUCOSAL IMMUNITY II. ORGANIZATION OF THE MUCOSAL IMMUNE SYSTEM A. Components of the Mucosal Immune System B. Induction of a Response C. Features of Mucosal Immunity D. Intraepithelial lymphocytes (IEL) III. IgA SYNTHESIS, STRUCTURE AND TRANSPORT IV. FUNCTIONS OF IgA AT MUCOSAL SURFACES A. Barrier Functions B. Intraepithelial Viral Neutralization C. Excretory Immunity D. Passive Immunity E. IgA Deficiency State V. MUCOSAL IMMUNIZATION VI. MUCOSAL TOLERANCE A. The Induction of Tolerance via Mucosal Sites B. The interaction Between Gut Bacteria and the Intestine I.