Pathology of Anal Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anatomy of the Rectum and Anal Canal
BASIC SCIENCE identify the rectosigmoid junction with confidence at operation. The anatomy of the rectum The rectosigmoid junction usually lies approximately 6 cm below the level of the sacral promontory. Approached from the distal and anal canal end, however, as when performing a rigid or flexible sigmoid- oscopy, the rectosigmoid junction is seen to be 14e18 cm from Vishy Mahadevan the anal verge, and 18 cm is usually taken as the measurement for audit purposes. The rectum in the adult measures 10e14 cm in length. Abstract Diseases of the rectum and anal canal, both benign and malignant, Relationship of the peritoneum to the rectum account for a very large part of colorectal surgical practice in the UK. Unlike the transverse colon and sigmoid colon, the rectum lacks This article emphasizes the surgically-relevant aspects of the anatomy a mesentery (Figure 1). The posterior aspect of the rectum is thus of the rectum and anal canal. entirely free of a peritoneal covering. In this respect the rectum resembles the ascending and descending segments of the colon, Keywords Anal cushions; inferior hypogastric plexus; internal and and all of these segments may be therefore be spoken of as external anal sphincters; lymphatic drainage of rectum and anal canal; retroperitoneal. The precise relationship of the peritoneum to the mesorectum; perineum; rectal blood supply rectum is as follows: the upper third of the rectum is covered by peritoneum on its anterior and lateral surfaces; the middle third of the rectum is covered by peritoneum only on its anterior 1 The rectum is the direct continuation of the sigmoid colon and surface while the lower third of the rectum is below the level of commences in front of the body of the third sacral vertebra. -

A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for High-Grade Anal Intraepithelial Neoplasia
Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for High-Grade Anal Intraepithelial Neoplasia Using The BARRX™ Anorectal Wand (Clinical Protocol 01-2017) AUGUST 31, 2017 Page 1 of 40 Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia PROTOCOL SIGNATURE PAGE I, , Principal Investigator at site , agree to conduct and follow this protocol: PROTOCOL 01-2017: A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for Anal Intraepithelial Neoplasia using the Barrx™ Anorectal Wand as written according to FDA guidelines. I understand that no deviations from the above protocol may be made without written permission from the Protocol Chair(s). _________________________________ _____________________ Signature Date (mm/dd/yyyy) Page 2 of 40 Protocol 01-2017: RFA for Anal Intraepithelial Neoplasia P ROT O COL S U M M ARY Title A Safety and Efficacy Trial of Circumferential Anal Canal Radiofrequency Ablation for Anal Intraepithelial Neoplasia using the Barrx™ Anorectal Wand Design Multi-center prospective trial involving up to 70 subjects Subject Population HIV-positive and HIV-negative subjects with intra-anal intraepithelial neoplasia (AIN) containing at least one high- grade squamous intraepithelial lesions (HSIL) involving the squamocolumnar junction (SCJ). Objective Assess the safety, and efficacy of circumferential radiofrequency ablation (RFA) to the anal canal using the FDA cleared Barrx™ Anorectal Wand to eradicate anal -

Rectum & Anal Canal
Rectum & Anal canal Dr Brijendra Singh Prof & Head Anatomy AIIMS Rishikesh 27/04/2019 EMBRYOLOGICAL basis – Nerve Supply of GUT •Origin: Foregut (endoderm) •Nerve supply: (Autonomic): Sympathetic Greater Splanchnic T5-T9 + Vagus – Coeliac trunk T12 •Origin: Midgut (endoderm) •Nerve supply: (Autonomic): Sympathetic Lesser Splanchnic T10 T11 + Vagus – Sup Mesenteric artery L1 •Origin: Hindgut (endoderm) •Nerve supply: (Autonomic): Sympathetic Least Splanchnic T12 L1 + Hypogastric S2S3S4 – Inferior Mesenteric Artery L3 •Origin :lower 1/3 of anal canal – ectoderm •Nerve Supply: Somatic (inferior rectal Nerves) Rectum •Straight – quadrupeds •Curved anteriorly – puborectalis levator ani •Part of large intestine – continuation of sigmoid colon , but lacks Mesentery , taeniae coli , sacculations & haustrations & appendices epiploicae. •Starts – S3 anorectal junction – ant to tip of coccyx – apex of prostate •12 cms – 5 inches - transverse slit •Ampulla – lower part Development •Mucosa above Houstons 3rd valve endoderm pre allantoic part of hind gut. •Mucosa below Houstons 3rd valve upto anal valves – endoderm from dorsal part of endodermal cloaca. •Musculature of rectum is derived from splanchnic mesoderm surrounding cloaca. •Proctodeum the surface ectoderm – muco- cutaneous junction. •Anal membrane disappears – and rectum communicates outside through anal canal. Location & peritoneal relations of Rectum S3 1 inch infront of coccyx Rectum • Beginning: continuation of sigmoid colon at S3. • Termination: continues as anal canal, • one inch below -
![Clinical Reviewer: [Joohee Lee ] STN: [125508/153 ]](https://docslib.b-cdn.net/cover/7770/clinical-reviewer-joohee-lee-stn-125508-153-127770.webp)
Clinical Reviewer: [Joohee Lee ] STN: [125508/153 ]
Clinical Reviewer: [Joohee Lee ] STN: [125508/153 ] Application sBLA Type STN 125508/153 CBER February 1, 2016 Received Date PDUFA Goal December 1, 2016 Date Division / DVRPA /OVRR Office Priority Review No Reviewer Joohee Lee Name(s) Review October 6, 2016 Completion Date / Stamped Date Supervisory Concurrence Lucia Lee, M.D., Team Leader, CRB1 Jeff Roberts, M.D., Chief, CRB1 Applicant Merck Established Human Papillomavirus 9-valent Vaccine, Recombinant Name (Proposed) Gardasil 9 Trade Name Pharmacologic Vaccine Class Page i Clinical Reviewer: [Joohee Lee ] STN: [125508/153 ] Formulation(s), A 0.5 mL dose contains: including Recombinant virus-like particles (VLPs) of the major capsid (L1) Adjuvants, etc protein of HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58)* adsorbed on preformed aluminum-containing adjuvant (Amorphous Aluminum Hydroxyphosphate Sulfate or AAHS) *Amounts of HPV type L1 protein are as follows: 30 mcg/40mcg/60mcg/40mcg/20mcg/20mcg/20mcg/20mcg/20mcg, respectively. Dosage 0.5-mL suspension for intramuscular injection as a single-dose Form(s) and vial and prefilled syringe Route(s) of Administration Dosing Two-dose regimen: 0 and 6 to 12 months Regimen A 3- dose regimen (0, 2 and 6 months) is approved for use in individuals 9 through 26 years of age (STN 125508/0) Page ii Clinical Reviewer: [Joohee Lee ] STN: [125508/153 ] Indication(s) and Intended This supplement introduces a 2-dose regimen in addition to the Population(s) licensed 3-dose regimen. The intended population for the 2- dose regimen is girls and boys 9 through 14 years of age. -

2. Bilateral Cleft Anatomy 19
BILATERAL CLEFT ANATOMY IS ATTACHED TO THE SINGLE CLEFT THE PREMAXILLA NORMALLY ROTATED OUTWARD MAXILLA ON ONE SIDE AND THIS ENTIRE COMPONENT IS THE CLEFT SIDE MAXILLA IN AN VARYING DEGREES FROM ASYMMETRICAL DIFFERENT DISTORTION DOUBLE CLEFTS PRESENT AN ENTIRELY CONFIGURA TION IN THE COMPLETE BILATERAL CLEFT THE PREMAXILLA IS UNATTACHED THREE WHICH TO EITHER MAXILLA THUS THERE ARE SEPARATE COMPONENTS IN THEIR DISTORTION THE MAXILLAE ARE MORE OR LESS SYMMETRICAL TWO WHILE THE ARE USUALLY EQUAL TO EACH OTHER IN SIZE AND POSITION FORWARD ITS IN CENTRAL PREMAXILLARY ELEMENT PROCEEDS ON OWN WITHIN ITSELF FOR DIFFERENT DEGREES BUT WITH SYMMETRY EXCEPT IJI POSSIBLE DEVIATION FRONTONASAL THE COMPLETE SEPARATION OF THE CENTRAL COMPONENT OF PROLABIUM AND PREMAXILLA FROM THE LATERAL MAXILLARY SEGMENTS THE VASCULAR ABNORMALLY INFLUENCES NOSE PHILTRUM MUSCULATURE AND OF ALL THREE ELEMENTS ITY NERVE SUPPLY GROWTH DEVELOPMENT WHERE THE CLEFT IS INCOMPLETE ON BOTH SIDES THE DEFORMITY IS LESS AND IS STILL SYMMETRICAL IN SUCH CASE THERE IS USUALLY MORE OR LESS INTACT ALVEOLUS AND LITTLE OR NO PROTRUSION OF THE PRE THE MAXILLA THE COLUMELLA IS LIKELY TO BE LONGER THAN IN COMPLETE CLEFT BUT NOT OF NORMAL LENGTH SOMETIMES SOMETIMES THE DEGREE OF CLEFT VARIES ON EACH SIDE SIDE THE INCOMPLETENESS SHOWS AS ONLY THE SLIGHTEST NOTCH ON ONE SIDE OR THERE CLEFT ON THE OPPOSITE AND HALFWAY OR THREEQUARTER ON THE CLEFT ONE SIDE AND AN INCOMPLETE ONE CAN BE COMPLETE ON OF THE EXASPERATING ASPECT OTHER WHICH CONDITION EXAGGERATES THE ROTATION OF THE IN THE AND NOSE -
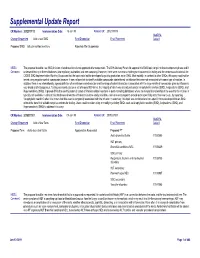
Detail Report
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

48 Anal Canal
Anal Canal The rectum is a relatively straight continuation of the colon about 12 cm in length. Three internal transverse rectal valves (of Houston) occur in the distal rectum. Infoldings of the submucosa and the inner circular layer of the muscularis externa form these permanent sickle- shaped structures. The valves function in the separation of flatus from the developing fecal mass. The mucosa of the first part of the rectum is similar to that of the colon except that the intestinal glands are slightly longer and the lining epithelium is composed primarily of goblet cells. The distal 2 to 3 cm of the rectum forms the anal canal, which ends at the anus. Immediately proximal to the pectinate line, the intestinal glands become shorter and then disappear. At the pectinate line, the simple columnar intestinal epithelium makes an abrupt transition to noncornified stratified squamous epithelium. After a short transition, the noncornified stratified squamous epithelium becomes continuous with the keratinized stratified squamous epithelium of the skin at the level of the external anal sphincter. Beneath the epithelium of this region are simple tubular apocrine sweat glands, the circumanal glands. Proximal to the pectinate line, the mucosa of the anal canal forms large longitudinal folds called rectal columns (of Morgagni). The distal ends of the rectal columns are united by transverse mucosal folds, the anal valves. The recess above each valve forms a small anal sinus. It is at the level of the anal valves that the muscularis mucosae becomes discontinuous and then disappears. The submucosa of the anal canal contains numerous veins that form a large hemorrhoidal plexus. -

NCCN Guidelines for Patients Anal Cancer
NCCN GUIDELINES FOR PATIENTS® 2021 Anal Cancer Presented with support from: Available online at NCCN.org/patients Ü Anal Cancer It's easy to get lost in the cancer world Let NCCN Guidelines for Patients® be your guide 9 Step-by-step guides to the cancer care options likely to have the best results 9 Based on treatment guidelines used by health care providers worldwide 9 Designed to help you discuss cancer treatment with your doctors NCCN Guidelines for Patients® Anal Cancer, 2021 1 About National Comprehensive Cancer Network® NCCN Guidelines for Patients® are developed by the National Comprehensive Cancer Network® (NCCN®) NCCN Clinical Practice NCCN Guidelines NCCN Guidelines in Oncology for Patients (NCCN Guidelines®) 9 An alliance of leading 9 Developed by doctors from 9 Present information from the cancer centers across the NCCN cancer centers using NCCN Guidelines in an easy- United States devoted to the latest research and years to-learn format patient care, research, and of experience 9 For people with cancer and education 9 For providers of cancer care those who support them all over the world Cancer centers 9 Explain the cancer care that are part of NCCN: 9 Expert recommendations for options likely to have the NCCN.org/cancercenters cancer screening, diagnosis, best results and treatment Free online at Free online at NCCN.org/patientguidelines NCCN.org/guidelines and supported by funding from NCCN Foundation® These NCCN Guidelines for Patients are based on the NCCN Guidelines® for Anal Cancer, Version 1.2021 — February 16, 2021. © 2021 National Comprehensive Cancer Network, Inc. All rights reserved. -

The Digestive System Overview of the Digestive System • Organs Are Divided Into Two Groups the Alimentary Canal and Accessory
C H A P T E R 23 The Digestive System 1 Overview of the Digestive System • Organs are divided into two groups • The alimentary canal • Mouth, pharynx, and esophagus • Stomach, small intestine, and large intestine (colon) • Accessory digestive organs • Teeth and tongue • Gallbladder, salivary glands, liver, and pancreas 2 The Alimentary Canal and Accessory Digestive Organs Mouth (oral cavity) Parotid gland Tongue Sublingual gland Salivary glands Submandibular gland Esophagus Pharynx Stomach Pancreas (Spleen) Liver Gallbladder Transverse colon Duodenum Descending colon Small intestine Jejunum Ascending colon Ileum Cecum Large intestine Sigmoid colon Rectum Anus Vermiform appendix Anal canal Figure 23.1 3 1 Digestive Processes • Ingestion • Propulsion • Mechanical digestion • Chemical digestion • Absorption • Defecation 4 Peristalsis • Major means of propulsion • Adjacent segments of the alimentary canal relax and contract Figure 23.3a 5 Segmentation • Rhythmic local contractions of the intestine • Mixes food with digestive juices Figure 23.3b 6 2 The Peritoneal Cavity and Peritoneum • Peritoneum – a serous membrane • Visceral peritoneum – surrounds digestive organs • Parietal peritoneum – lines the body wall • Peritoneal cavity – a slit-like potential space Falciform Anterior Visceral ligament peritoneum Liver Peritoneal cavity (with serous fluid) Stomach Parietal peritoneum Kidney (retroperitoneal) Wall of Posterior body trunk Figure 23.5 7 Mesenteries • Lesser omentum attaches to lesser curvature of stomach Liver Gallbladder Lesser omentum -

What Is Anal Cancer?
cancer.org | 1.800.227.2345 About Anal Cancer Overview and Types If you've been diagnosed with anal cancer or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Is Anal Cancer? Research and Statistics See the latest estimates for new cases of anal cancer and deaths in the US and what research is currently being done. ● Key Statistics for Anal Cancer ● What’s New in Anal Cancer Research? What Is Anal Cancer? Anal cancer is a type of cancer that starts in the anus. Cancer starts when cells in the body begin to grow out of control. To learn more about how cancers start and spread, see What Is Cancer?1 Normal structure and function of the anus 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 The anus is the opening at the lower end of the intestines. It's where the end of the intestines connect to the outside of the body. As food is digested, it passes from the stomach to the small intestine. It then moves from the small intestine into the main part of the large intestine (called the colon). The colon absorbs water and salt from the digested food. The waste matter that's left after going through the colon is known as feces or stool. Stool is stored in the last part of the large intestine, called the rectum. From there, stool is passed out of the body through the anus as a bowel movement. Gastrointestinal system (GI system) Structures of the anus The anus is connected to the rectum by the anal canal. -

Progress Report Anal Continence
Gut: first published as 10.1136/gut.12.10.844 on 1 October 1971. Downloaded from Gut, 1971, 12, 844-852 Progress report Anal continence Anal continence depends on an adaptable barrier formed at the ano-rectal junction and in the anal canal by a combination of forces. These are due in part to the configuration of the region and in part to the action of muscles. The forces are activated in response to sensory information obtained from the rectum and the anal canal. In order to understand some of the concepts of the mechanism of anal continence, some of the features of the anatomy and physiology of the region will be discussed. Anatomy (Fig. 1) The lumen of the rectum terminates at the pelvic floor and is continued, downwards and posteriorly, as the anal canal, passing through the levator ani muscle sheet and surrounded by the internal and external anal sphincters. The anal canal is 2.5 to 5 cm in length and 3 cm in diameter when distended. The axis of the rectum forms almost a right angle (average 820) with the axis of the anal canal. It has been established by radiological studies that the anal canal is an antero-posterior slit in the resting state.' The former concept of http://gut.bmj.com/ the anal canal being surrounded successively craniocaudally by the internal anal sphincter and then the external anal sphincter has been replaced by the knowledge that the two muscles overlap to a considerable extent with the external sphincter wrapped round the internal sphincter2'3. -

Infectious Gastroenteritis Generalised Anxiety Disorder HIV Smoking Associated Cancers
BEST PRACTICE 25 DECEMBER 2009 Infectious gastroenteritis Generalised anxiety disorder HIV bpac nz Smoking associated cancers better medicin e Editorial Team Tony Fraser Professor Murray Tilyard We would like to acknowledge the following people for Clinical Advisory Group their guidance and expertise in developing this edition: Michele Cray Dr Shaun Costello, Dunedin Serena Curtis-Lemuelu Dr Edward Coughlan, Christchurch Dr Rosemary Ikram Professor Tony Dowell, Wellington Dr Cam Kyle Dr Rosemary Ikram, Christchurch Dr Chris Leathart Mr William Pearce, Christchurch Dr Lynn McBain Dr Alan Pithie, Christchurch Adam McRae Dr Gabrielle Ruben, Wellington Janet Maloney-Moni Assoc. Professor Mark Thomas, Auckland Dr Peter Moodie Dr Robyn Toomath, Wellington Associate Professor Jim Reid Dr Neil Whittaker, GP Reviewer, Nelson Associate Professor David Reith Assoc. Professor Michael Williams, Dunedin Professor Murray Tilyard Programme Development Team Rachael Clarke Peter Ellison Best Practice Journal (BPJ) Rebecca Harris Julie Knight ISSN 1177-5645 Noni Richards BPJ, Issue 25, December 2009 Dr Tom Swire Dr AnneMarie Tangney nz Dr Sharyn Willis BPJ is published and owned by bpac Ltd Dave Woods Level 8, 10 George Street, Dunedin, New Zealand. Report Development Team Bpacnz Ltd is an independent organisation that promotes health Justine Broadley care interventions which meet patients’ needs and are evidence Todd Gillies based, cost effective and suitable for the New Zealand context. Lana Johnson We develop and distribute evidence based resources which describe, facilitate and help overcome the barriers to best Web practice. Gordon Smith Bpacnz Ltd is currently funded through contracts with PHARMAC Design and DHBNZ. Michael Crawford Bpacnz Ltd has five shareholders: Procare Health, South Link Management and Administration Health, IPAC, the University of Otago and Pegasus Health.