Pro Partes', Which Does Not Seem Useful
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Invasive Alien Plants an Ecological Appraisal for the Indian Subcontinent
Invasive Alien Plants An Ecological Appraisal for the Indian Subcontinent EDITED BY I.R. BHATT, J.S. SINGH, S.P. SINGH, R.S. TRIPATHI AND R.K. KOHL! 019eas Invasive Alien Plants An Ecological Appraisal for the Indian Subcontinent FSC ...wesc.org MIX Paper from responsible sources `FSC C013604 CABI INVASIVE SPECIES SERIES Invasive species are plants, animals or microorganisms not native to an ecosystem, whose introduction has threatened biodiversity, food security, health or economic development. Many ecosystems are affected by invasive species and they pose one of the biggest threats to biodiversity worldwide. Globalization through increased trade, transport, travel and tour- ism will inevitably increase the intentional or accidental introduction of organisms to new environments, and it is widely predicted that climate change will further increase the threat posed by invasive species. To help control and mitigate the effects of invasive species, scien- tists need access to information that not only provides an overview of and background to the field, but also keeps them up to date with the latest research findings. This series addresses all topics relating to invasive species, including biosecurity surveil- lance, mapping and modelling, economics of invasive species and species interactions in plant invasions. Aimed at researchers, upper-level students and policy makers, titles in the series provide international coverage of topics related to invasive species, including both a synthesis of facts and discussions of future research perspectives and possible solutions. Titles Available 1.Invasive Alien Plants : An Ecological Appraisal for the Indian Subcontinent Edited by J.R. Bhatt, J.S. Singh, R.S. Tripathi, S.P. -

In Vitro Free Radical Scavenging and Thrombolytic Activities of Bangladeshi Aquatic Plant Aponogeton Undulatus Roxb
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by BRAC University Institutional Repository Global Journal of Pharmacology 5 (1): 27-32, 2011 ISSN 1992-0075 © IDOSI Publications, 2011 In vitro Free Radical Scavenging and Thrombolytic Activities of Bangladeshi Aquatic Plant Aponogeton undulatus Roxb 1Nargis S. Chowdhury, 23M. Badrul Alam, A.S.M. Tanbirul Haque, 24Ronok Zahan, M. Ehsanul H. Mazumder and 5M. Ekramul Haque 1Department of Pharmacy, Manarat International University, Dhaka, Bangladesh 2Department of Pharmacy, Atish Dipankar University of Science & Technology, Banani, Dhaka, Bangladesh 3Department of Agribusiness, Atish Dipankar University of Science & Technology, Banani, Dhaka, Bangladesh 4Discipline of Biomedical Science, Faculty of Medicine, Cumberland Campus, University of Sydney, Australia 5Department of Pharmacy, BRAC University, Mohakhali, Dhaka, Bangladesh Abstract: Investigation with the crude methanolic extract of Aponogeton undulatus was carried out to evaluate its possible antioxidant and thrombolysis activity. In DPPH free radical scavenging assay, the extract exhibited potent antioxidant activity with a IC50 values of 2.43±1.06 µg/ml while in ascorbic acid, the value become 2.14±0.11 µg/ml. In thrombolytic activity using in vitro clot lysis assay method, the crude methanolic extract was found to have significant (p<0.001) thrombolytic activity at a dose of 10 mg/ml with a miximum effect of 20.23±1.56% while the standard streptokinase showed 46.13±3.87%. The extract was also investigated for its antibacterial and toxic potentiality using agar diffusion and Brine Shrimp lethality bioassay, respectively. The highest antibacterial effect was shown against Bacillus cereus (zone of inhibition 12±0.65 mm) followed by Escherichia coli (zone of inhibition 10±0.71 mm). -

Low Risk Aquarium and Pond Plants
Plant Identification Guide Low-risk aquarium and pond plants Planting these in your pond or aquarium is environmentally-friendly. Glossostigma elatinoides, image © Sonia Frimmel. One of the biggest threats to New Zealand’s waterbodies is the establishment and proliferation of weeds. The majority of New Zealand’s current aquatic weeds started out as aquarium and pond plants. To reduce the occurrence of new weeds becoming established in waterbodies this guide has been prepared to encourage the use of aquarium and pond plants that pose minimal risk to waterbodies. Guide prepared by Dr John Clayton, Paula Reeves, Paul Champion and Tracey Edwards, National Centre of Aquatic Biodiversity and Biosecurity, NIWA with funding from the Department of Conservation. The guides will be updated on a regular basis and will be available on the NIWA website: www.niwa.co.nz/ncabb/tools. Key to plant life-forms Sprawling marginal plants. Grow across the ground and out over water. Pond plants Short turf-like plants. Grow in shallow water on the edges of ponds and foreground of aquariums. Includes very small plants (up to 2-3 cm in height). Most species can grow both submerged (usually more erect) and emergent. Pond and aquarium plants Tall emergent plants. Can grow in water depths up to 2 m deep depending on the species. Usually tall reed-like plants but sometimes with broad leaves. Ideal for deeper ponds. Pond plants Free floating plants. These plants grow on the water surface and are not anchored to banks or bottom substrates. Pond and aquarium plants Floating-leaved plants. Water lily-type plants. -

SOME OBSERVATIONS on the IMPORTANCE of Aponogeton Rigidifolius in ENDEMIC FISH BREEDING HABITATS Iitloya
Kfflhories and Aquatic Resources, 2004 SO M E OBSERVATIONS ON THE IMPORTANCE OF Aponogeton rigidifolius IN ENDEMIC FISH BREEDING HABITATS A.S.L.E. Corea,jM.S.S. Jayasekara and N.N.E Cooray Notional Aquatic Resources Research and Development agency (NARA), Crow Island, Mattakkuliya, Colombo 15, Sri Lanka Aponogeton rigidifolius is an endemic aquatic plant with thin long laiives extending to over 0.5m in length. This species is mainly found in ilroams in the wet zone. Fish species associated with A.' rigidifolius were sampled using a drag net made of mosquito netting material. The net was dragged 10 times And the mean number of fish caught per net was recorded. In areas with a dShse population of A. rigidifolius (82 ± 6.33 plants / m2) with leaf size 46 ± 10.2 cm, the number of R. vaterifloris collected per net sample was 32 ± 5.6. The size range of fish collected was 0.8 - 3.0 cm. In the areas with dense patches of A. rigidifolius, the dominant species was R. vaterifloris. The number of R. vaterifloris caught per net sample in areas where there were no A. rigidifolius was <10, and the size range of fish in these areas was 1.4 * 2,8 cm. The fry which were <1.2 cm in total length were found only in siBIOciation with A. rigidifolius. They were found in these habitats throughout lb® year. A total of 600 plants of A. rigidifolius was introduced to a site of 10 m® In the same stream devoid of vegetation. At the point of introduction two inclemic species, namely Belontia signata (size 1.5 - 6.0 cm, 14±2.33 fish / hffit) and Puntius titteya (size 1.5 - 3.0 cm, 6+1.1 fish / net) were observed at lb® site of introduction. -

Aponogeton Pollen from the Cretaceous and Paleogene of North America and West Greenland: Implications for the Origin and Palaeobiogeography of the Genus☆
Review of Palaeobotany and Palynology 200 (2014) 161–187 Contents lists available at ScienceDirect Review of Palaeobotany and Palynology journal homepage: www.elsevier.com/locate/revpalbo Research paper Aponogeton pollen from the Cretaceous and Paleogene of North America and West Greenland: Implications for the origin and palaeobiogeography of the genus☆ Friðgeir Grímsson a,⁎, Reinhard Zetter a, Heidemarie Halbritter b, Guido W. Grimm c a University of Vienna, Department of Palaeontology, Althanstraße 14 (UZA II), Vienna, Austria b University of Vienna, Department of Structural and Functional Botany, Rennweg 14, Vienna, Austria c Swedish Museum of Natural History, Department of Palaeobiology, Box 50007, 10405 Stockholm, Sweden article info abstract Article history: The fossil record of Aponogeton (Aponogetonaceae) is scarce and the few reported macrofossil findings are in Received 15 January 2013 need of taxonomic revision. Aponogeton pollen is highly diagnostic and when studied with light microscopy Received in revised form 4 September 2013 (LM) and scanning electron microscopy (SEM) it cannot be confused with any other pollen types. The fossil Accepted 22 September 2013 Aponogeton pollen described here represent the first reliable Cretaceous and Eocene records of this genus world- Available online 3 October 2013 wide. Today, Aponogeton is confined to the tropics and subtropics of the Old World, but the new fossil records show that during the late Cretaceous and early Cenozoic it was thriving in North America and Greenland. The Keywords: Alismatales late Cretaceous pollen record provides important data for future phylogenetic and phylogeographic studies Aponogetonaceae focusing on basal monocots, especially the Alismatales. The Eocene pollen morphotypes from North America aquatic plant and Greenland differ in morphology from each other and also from the older Late Cretaceous North American early angiosperm pollen morphotype, indicating evolutionary trends and diversification within the genus over that time period. -

Dr. S. R. Yadav
CURRICULUM VITAE NAME : SHRIRANG RAMCHANDRA YADAV DESIGNATION : Professor INSTITUTE : Department of Botany, Shivaji University, Kolhapur 416004(MS). PHONE : 91 (0231) 2609389, Mobile: 9421102350 FAX : 0091-0231-691533 / 0091-0231-692333 E. MAIL : [email protected] NATIONALITY : Indian DATE OF BIRTH : 1st June, 1954 EDUCATIONAL QUALIFICATIONS: Degree University Year Subject Class B.Sc. Shivaji University 1975 Botany I-class Hons. with Dist. M.Sc. University of 1977 Botany (Taxonomy of I-class Bombay Spermatophyta) D.H.Ed. University of 1978 Education methods Higher II-class Bombay Ph.D. University of 1983 “Ecological studies on ------ Bombay Indian Medicinal Plants” APPOINTMENTS HELD: Position Institute Duration Teacher in Biology Ruia College, Matunga 16/08/1977-15/06/1978 JRF (UGC) Ruia College, Matunga 16/06/1978-16/06/1980 SRF (UGC) Ruia College, Matunga 17/06/1980-17/06/1982 Lecturer J.S.M. College, Alibag 06/12/1982-13/11/1984 Lecturer Kelkar College, Mulund 14/11/1984-31/05/1985 Lecturer Shivaji University, Kolhapur 01/06/1985-05/12/1987 Sr. Lecturer Shivaji University, Kolhapur 05/12/1987-31/01/1993 Reader and Head Goa University, Goa 01/02/1993-01/02/1995 Sr. Lecturer Shivaji University, Kolhapur 01/02/1995-01/12/1995 Reader Shivaji University, Kolhapur 01/12/1995-05/12/1999 Professor Shivaji University, Kolhapur 06/12/1999-04/06/2002 Professor University of Delhi, Delhi 05/06/2002-31/05/2005 Professor Shivaji University, Kolhapur 01/06/2005-31/05/2014 Professor & Head Department of Botany, 01/06/2013- 31/05/2014 Shivaji University, Kolhapur Professor & Head Department of Botany, 01/08/ 2014 –31/05/ 2016 Shivaji University, Kolhapur UGC-BSR Faculty Department of Botany, Shivaji 01/06/2016-31/05/2019 Fellow University, Kolhapur. -

(19) United States (12) Patent Application Publication (10) Pub
US 20060030489A1 (19) United States (12) Patent Application Publication (10) Pub. N0.: US 2006/0030489 A1 Northcott et al. (43) Pub. Date: Feb. 9, 2006 (54) AQUATIC PLANT PRODUCT AND METHOD Publication Classi?cation FOR MAKING GROWTH-SUSTAINING PLANT MATRIX (51) Int. Cl. A01N 39/02 (2006.01) (76) Inventors: Donald Owen Northcott, Cornwall A01H 9/00 (2006.01) (CA); Mark O. Hamran, Tea, SD (US) (52) Us. 01. .......................................... .. 504/323; 800/295 Correspondence Address: (57) ABSTRACT MARIO D. THERIAULT The invention consists of an aquatic plant product for resale, 812 HWY. 101 NASONWORTH having an extended shelf life, and a method for making the FREDERICTON, NB E3C 2B5 (CA) aquatic plant product. The aquatic plant product is made of a sealed container containing aquatic-plant-groWth-sustain (21) Appl. No.: 11/193,503 ing medium therein and an aquatic plant planted in that aquatic-plant-groWth-sustaining medium. The aquatic-plant (22) Filed: Aug. 1, 2005 groWth-sustaining medium contains a root-support material; at least one vitamin; sucrose; agar; distilled Water; micro Related US. Application Data nutrients containing iron; at least one hormone selected from a group consisting of auxin, naphthleneacetic acid, indole (60) Provisional application No. 60/599,985, ?led on Aug. 3-butric acid, cytokine, 2-isopentyladenine, 6 BenZylami 9, 2004. nopurine and any combination thereof. US 2006/0030489 A1 Feb. 9, 2006 AQUATIC PLANT PRODUCT AND METHOD FOR [0012] e) distilled Water; and MAKING GROWTH-SUSTAINING PLANT MATRIX [0013] f) at least one hormone selected from the list [0001] This application claims the bene?ts of provisional consisting of auxin, naphthleneacetic acid, indole-3-butric application Ser. -
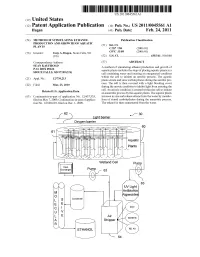
(12) Patent Application Publication (10) Pub. No.: US 2011/0045561 A1 Hagen (43) Pub
US 2011 0045561A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0045561 A1 Hagen (43) Pub. Date: Feb. 24, 2011 (54) METHOD OF STIMULATING ETHANOL Publication Classification PRODUCTION AND GROWTH OF AOUATIC PLANTS (51) Int. Cl. CI2P 7/06 (2006.01) (76) Inventor: Tony A. Hagen, Sioux Falls, SD C07C3L/08 (2006.01) (US) (52) U.S. Cl. ......................................... 435/161; 568/840 Correspondence Address: (57) ABSTRACT ED A method of stimulating ethanol production and growth of soUX FALLS SD 57.109 (US aquatic plants includes the steps of placing aquatic plants in a 9 (US) cell containing water and creating an oxygenated condition within the cell to initiate an aerobic process. The aquatic (21) Appl. No.: 12/730,213 plants create and store carbohydrates during the aerobic pro 1-1. cess. The cell is then covered with a light blocking cover (22) Filed: Mar. 23, 2010 during the anoxic condition to inhibit light from entering the O O cell. An anoxic condition is created within the cell to initiate Related U.S. Application Data an anaerobic process by the aquatic plants. The aquatic plants (63) Continuation-in-part of application No. 12/437.333, increase in size and release ethanol into the water by metabo filed on May 7, 2009, Continuation-in-part of applica lism of stored carbohydrates during the anaerobic process. tion No. 12/628,601, filed on Dec. 1, 2009. The ethanol is then sequestered from the water. u- 30 Light barrier 1 D Oxygen barrier 60 61 Aq uatic Plants -1 -/N- ^- ----- Wetland Cell eat Exchanger Pump 63 :::::::::::: (Or UV Light CO Antibiotics Algaecides Corderse Patent Application Publication Feb. -

DAAS HAP March 2017.Pdf
Danbury Area Aquarium Society Horticulture Awards Program Purpose: The purpose of the Horticultural Award Program (HAP) is to promote the keeping and propagation of aquatic plants, aid in the recognition of the species, encourage research, through the growth and propagation of different species, and recognize achievements of individuals through awards. Aquatic Plant Defined: An aquatic plant is one with a submerged or floating form, as a normal occurrence, at some time during the course of any one complete growing season. The HAP Committee: The President shall appoint the HAP Chair, and the HAP Chair shall appoint the remaining members as necessary. Function of the HAP Committee: To oversee and enforce all rules and regulations governing the HAP, awarding points to qualifying members, maintaining records and presenting awards. The HAP rules and regulations shall be reviewed and revised when necessary. HAP Checkers: Any person on the HAP committee may verify the species of a submitted plant and any flowerings or sexual propagation, with the HAP chair having final approval. The HAP Chair reserves the right to reject stunted, algae covered, or unhealthy plants. Noxious and Invasive Plants: The importation, transportation, sale, purchase, possession, cultivation or distribution of a number of invasive plants including the following aquatic and wetland plants is currently prohibited by Rhode Island, Massachusetts or Connecticut laws. Since one of the goals of DAAS includes conservation of native resources, the following plants are excluded -

Morphometric Analysis Reveals a New Species of Aponogeton (Aponogetonaceae) in Sri Lanka
Phytotaxa 275 (3): 243–262 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2016 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.275.3.3 Morphometric analysis reveals a new species of Aponogeton (Aponogetonaceae) in Sri Lanka CHAPA G. MANAWADUGE1, DEEPTHI YAKANDAWALA1* & DONALD H. LES2 1Department of Botany, University of Peradeniya, Peradeniya, Sri Lanka 2Department of Ecology & Evolutionary Biology, University of Connecticut, Storrs, CT, USA *Corresponding author: [email protected], [email protected] Abstract A morphometric analysis of Sri Lankan Aponogeton was performed in order to clarify species delimitations and to facilitate their identifications by comparing states coded for 61 vegetative and reproductive characters. Both cluster analysis and principal coordinate analysis resolved five discrete phenetic groups within the genus in the island. Four of these clusters cor- responded well with the four previously reported species (A. rigidifolius, A. jacobsenii, A. crispus and A. natans) whereas a fifth cluster delimited a new Aponogeton species, A. dassanayakei, from the wet lowland regions of Sri Lanka. These analy- ses have disclosed more useful characters for field identification of the species. Key Words: Aponogeton dassanayakei, Aquatic plants, Cluster analysis, Morphology, Phenetics Introduction Despite its small size (65,610 km2), Sri Lanka contains a rich and diverse flora. The island is known to harbor 3,154 flowering plant species (MOE 2012) but potentially contains many that remain undescribed. About 370 species (<12% of total) represent aquatic or wetland plants, wherein 205 (6% of total) are monocots, which include the genus Aponogeton Linnaeus f. -

Expert Consultation on Promotion of Medicinal and Aromatic Plants in the Asia-Pacific Region
Expert Consultation on Promotion of Medicinal and Aromatic Plants in the Asia-Pacific Region Bangkok, Thailand 2-3 December, 2013 PROCEEDINGS Editors Raj Paroda, S. Dasgupta, Bhag Mal, S.P. Ghosh and S.K. Pareek Organizers Asia-Pacific Association of Agricultural Research Institutions (APAARI) Food and Agriculture Organization of the United Nations - Regional Office for Asia and the Pacific (FAO RAP) Citation : Raj Paroda, S. Dasgupta, Bhag Mal, S.P. Ghosh and S.K. Pareek. 2014. Expert Consultation on Promotion of Medicinal and Aromatic Plants in the Asia-Pacific Region: Proceedings, Bangkok, Thailand; 2-3 December, 2013. 259 p. For copies and further information, please write to: The Executive Secretary Asia-Pacific Association of Agricultural Research Institutions (APAARI) C/o Food and Agriculture Organization of the United Nations Regional Office for Asia & the Pacific 4th Floor, FAO RAP Annex Building 201/1 Larn Luang Road, Klong Mahanak Sub-District Pomprab Sattrupai District, Bangkok 10100, Thailand Tel : (+662) 282 2918 Fax : (+662) 282 2919 E-mail: [email protected] Website : www.apaari.org Printed in July, 2014 The Organizers APAARI (Asia-Pacific Association of Agricultural Research Institutions) is a regional association that aims to promote the development of National Agricultural Research Systems (NARS) in the Asia-Pacific region through inter-regional and inter-institutional cooperation. The overall objectives of the Association are to foster the development of agricultural research in the Asia- Pacific region so as to promote the exchange of scientific and technical information, encourage collaborative research, promote human resource development, build up organizational and management capabilities of member institutions and strengthen cross-linkages and networking among diverse stakeholders. -

Isolation, Identification and Pharmacological Activities of Endophytic Fungi from Aponogeton Undulatus Roxb
Pharmacology & Pharmacy, 2020, 11, 350-361 https://www.scirp.org/journal/pp ISSN Online: 2157-9431 ISSN Print: 2157-9423 Isolation, Identification and Pharmacological Activities of Endophytic Fungi from Aponogeton undulatus Roxb. Nargis Sultana Chowdhury1*, Farhana Farjana1, Md. Hossain Sohrab2 1Manarat International University, Dhaka, Bangladesh 2Pharmaceutical Sciences Research Division (PSRD), BCSIR Laboratories, Dhaka, Bangladesh How to cite this paper: Chowdhury, N.S., Abstract Farjana, F. and Sohrab, Md.H. (2020) Isola- tion, Identification and Pharmacological Acti- The objective of this study is to isolate, identify and investigate the pharmacol- vities of Endophytic Fungi from Aponoge- ogical activities of the endophytic fungi from an aquatic plant Aponogeton ton undulatus Roxb. Pharmacology & Phar- undulatus Roxb. (A. undulatus). Endophytic fungi were isolated and identi- macy, 11, 350-361. fied based on morphological characters. The molecular identification of the https://doi.org/10.4236/pp.2020.1112028 fungal isolates was performed using by analyzing the DNA sequence based on Received: November 5, 2020 mega BLAST program. Spectrums of antibacterial and antifungal activities Accepted: December 13, 2020 were studied by Agar diffusion methods. Extracts of the endophytic fungal Published: December 16, 2020 strains isolated from the plant A. undulatus were screened for probable cyto- Copyright © 2020 by author(s) and toxic activities using brine shrimp lethality bioassay. Six endophytic fungi, Scientific Research Publishing Inc. namely AULE-1, AULE-2, AULE-3, AURE-1, AURE-3 and AURE-4 were This work is licensed under the Creative isolated and purified from the leaves and roots of the plant such as strains Commons Attribution International AULE-1 as Trichooderma sp., AULE-2 as Carvularia sp.