Draft FSANZ Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Great Food, Great Stories from Korea
GREAT FOOD, GREAT STORIE FOOD, GREAT GREAT A Tableau of a Diamond Wedding Anniversary GOVERNMENT PUBLICATIONS This is a picture of an older couple from the 18th century repeating their wedding ceremony in celebration of their 60th anniversary. REGISTRATION NUMBER This painting vividly depicts a tableau in which their children offer up 11-1541000-001295-01 a cup of drink, wishing them health and longevity. The authorship of the painting is unknown, and the painting is currently housed in the National Museum of Korea. Designed to help foreigners understand Korean cuisine more easily and with greater accuracy, our <Korean Menu Guide> contains information on 154 Korean dishes in 10 languages. S <Korean Restaurant Guide 2011-Tokyo> introduces 34 excellent F Korean restaurants in the Greater Tokyo Area. ROM KOREA GREAT FOOD, GREAT STORIES FROM KOREA The Korean Food Foundation is a specialized GREAT FOOD, GREAT STORIES private organization that searches for new This book tells the many stories of Korean food, the rich flavors that have evolved generation dishes and conducts research on Korean cuisine after generation, meal after meal, for over several millennia on the Korean peninsula. in order to introduce Korean food and culinary A single dish usually leads to the creation of another through the expansion of time and space, FROM KOREA culture to the world, and support related making it impossible to count the exact number of dishes in the Korean cuisine. So, for this content development and marketing. <Korean Restaurant Guide 2011-Western Europe> (5 volumes in total) book, we have only included a selection of a hundred or so of the most representative. -

Food Additives - Coloring Permitted in Thailand Report Categories: Sanitary/Phytosanitary/Food Safety Approved By: Mr
THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY Voluntary - Public Date: 1/26/2011 GAIN Report Number: TH1010 Thailand Post: Bangkok Food Additives - Coloring Permitted in Thailand Report Categories: Sanitary/Phytosanitary/Food Safety Approved By: Mr. John Wade, Agricultural Counselor Prepared By: Ms. Sukanya Sirikeratikul, Marketing Specialist Report Highlights: TH1010: This report provides a list of color additives permitted to be used in foods in Thailand. The report also includes relevant ministerial notifications on food additives and the set maximum permitted amount of each color additive to be used in specific food product. General Information: Food additives mean the substances which normally are not used as food or essential ingredients of food, whether or not such substances have nutritional benefits, but which are added for the benefits of production technology, food coloring, food flavoring, packing, storage or transport which may affect food quality or standard or property. They also include the substances not added to the food but contained in certain package in the same container with food for the purpose mentioned earlier i.e. moisture absorber, oxygen absorber, etc. In Thailand, food additives are specified as specifically-controlled food of which the quality or standards are defined. Use of food additives must follow the set objectives for specified kinds of food and maximum permissible quantity, food additive functional -

Going for the Plant-Based (Legen)Dairy Alternative?
Going for the plant-based (legen)dairy alternative? An exploratory study on consumer attitudes and purchase intentions towards plant-based dairy alternatives Master thesis within: Business Administration - Marketing Number of credits: 30 ECTS Program of study: Civilekonom Authors: Emma Rosenlöw & Tommie Hansson Tutor: Adele Berndt Jönköping May 2020 Master Thesis in Business Administration - Marketing Title: Going for the plant-based (legen)dairy alternative? An exploratory study on consumer attitudes and purchase intentions towards plant-based dairy alternatives Authors: Emma Rosenlöw & Tommie Hansson Tutor: Adele Berndt Date: May 18, 2020 Key terms: Attitude, Environmental concern, Greenhouse gas, Health consciousness, Perceived behavioral control, Plant-based dairy substitutes, Purchase intention, Subjective norms Abstract Global food production, and consequently consumption, contributes significantly to total greenhouse gas emissions. Hence, there is a need for a shift towards more environmentally friendly consumption patterns. This includes moving away from current levels of dairy consumption, where plant-based alternatives can serve as more environmentally friendly options. This research sheds light on an emerging product category, namely plant-based dairy alternatives, which can serve as options or substitutes for traditional dairy products. The purpose of this thesis is to explore consumer attitudes and purchase intentions towards plant- based dairy alternatives, as well as the factors that influence attitudes and intentions respectively. To achieve an in-depth understanding of the topic, this study is of qualitative nature, using an abductive approach and interpretive philosophy. The primary data is collected through interviews with 16 consumers in the selected target group. Further, this research has developed a modified theory of planned behavior (TPB), to add to current consumer behavior research. -

Soymilk Production Process Dos and Donts
SoymilkSoymilk productionproduction processprocess DOsDOs andand DONTsDONTs IgnaceIgnace DebruyneDebruyne PhDPhD, Marketing Manager American Soybean Association - Europe & Maghreb [email protected] 4th International Soyfoods Conference SOUTHERN AFRICAN SOYFOOD ASSOCIATION/ SUIDER-AFRIKAANSE SOJAVOEDSELVERENIGING 17 July 2002, Gallagher Estates, South Africa Different roads to a same product ♦Traditional process ♦Soybean ultramilling and extraction ♦Formulation based on soy protein isolates ♦Soybean extraction – variations on an old traditional process Soaking for 20 h; room temperature Grinding into Cooking for 30 min a slush in pressure cooker Traditional Extraction Asiatic soymilk process Tonyu (soy milk) Okara (soy fiber) Traditional, Asiatic soymilk process ♦Exists since thousands of years ♦Product preferably with strong beany taste – not adapted to Western taste pattern ♦Starting product for tofu (bean curd) ♦Perishable okara byproduct; can be used in other food products Soybean ultramilling and extraction ♦ Buhler; FSP; ... ♦ Ultramilling to <10 µm (1000 mesh) or < 30 µm (400 mesh) ♦ Readily dispersible products, or extra need for high pressure homogenisation ♦ No or limited fiber separation ♦ Risk for off flavor formation (full fat flour highly sensitive to oxidation) So Good soy milk composition: ♦ Filtered water, soy protein, maltodextrin, grape extract, sunflower Reconstituted oil, acidity regulators (potassium citrate, potassium and diglycerides of fatty acids), stabiliser (carrageenan), soymilk salt, zinc gluconate, -

Food Facts File: the Healing Arts Soy Bean Or Not Soy Bean That Is The
Jacqui Chiplin Course: Nutrition Consultant NC4 Food facts file: The Healing Arts Soy Bean or not Soy Bean that is the Question… What would Shakespeare have made of the soy product? Would he have pondered whether it would be better for it to exist or not because of the controversies or troubles that surround it as did Hamlet with his life? Not really likely although soy sauce had been imported to Europe by Dutch traders in the 17th Century. In today’s world, the evolutionary journey of the soy bean leaves us with this important question. The Chinese first cultivated the soy bean 3,000 years ago and in the early days it was known as ‘shu’. Since then other Asian countries like Korea, Japan and Singapore have been using soy products for many years. Interestingly though, it wasn’t until the early 1800s that soy beans arrived in the United States. And it wasn’t until the 1940’s that the farming of soybeans actually became popular. So why did it take until the 20th Century for the United States and Europe to adopt soy? A journal written in the early 20th Century pointed to the need for an alternative food supply because of an international shortage of food. ‘The demand which the international shortage of food has created for cheaply produced and easily obtainable sources of all nutrients, and particularly of suitable proteins and fats, has directed attention anew to the possibilities of the soy bean.’ Global population increase had become a concern in the Western world. So why choose soy and what is it? The Chinese found that ‘The bean turned out to be a very cheap source of protein, yielding much more by acreage than milk, eggs or meat, or other common crops.’ Soy is a legume with an edible bean and the composition of whole soy is ‘36% protein, 30% carbs, 20% fat, 9% crude fiber and 5% ash’. -

Vegan Cooking for Carnivores Let Me Start Off by Saying That I Am Not a Chef, and I Have Never Been to Cooking School
Vegan Cooking For Carnivores Let me start off by saying that I am not a chef, and I have never been to cooking school. I learned to cook by watching my parents cook dinner every night and soon developed a love for cooking and especially baking. When I became vegan 12 years ago, I did so because of my love for animals and I realized that I didn’t need to consume animal products to live a healthy life. I didn’t do it because I didn’t like the taste of meat. If someone were to come out with a vegan steak that actually tasted like steak, I would be first in line to buy it. Anyway my point is that becoming vegan didn’t mean that I wanted to live off beans and sprouts for the rest of my life. I started off by modifying recipes I had always used and then as I got more adventurous, and as more meat and dairy alternatives came on the market, I started coming up with new recipes. None of my friends and family are vegan, it’s just me and my husband, so when I have people over for dinner, I like to make sure that they come back. That means making meals that taste like food they are used to. I’ve had several occasions where people have come over for dinner and doubted the food was vegan. The introduction food I make is so much like the real thing that my guests actually think I’ve strayed and put meat or dairy in it. -

29 Vegetarian Recipes for You to Cook and Then Eat
No Meat, Some Potatoes The Cookbook 29 vegetarian recipes for you to cook and then eat By Jane Garfinkel Table of Contents Thoughts......................................................................................................3 Smoothies........................................................................................................4 Go-To Green Smoothie (V)......................................................................4 Peanut Butter Chocolate Smoothie (V)...................................................5 Strawberry Smoothie Bowl (V)................................................................6 Brunch..........................................................................................................7 Shakshuka................................................................................................7 Blueberry Pancakes with Sour Cream Maple Topping..............................8 Spinach and Mushroom Quiche...............................................................9 Bowl Food...................................................................................................10 Tomato Soup..........................................................................................10 Chickpea Sweet Potato Curry(V)............................................................11 Mushroom Broth Ramen........................................................................12 Lentil and Sweet Potato Soup (V)...........................................................13 Instant Pot Chili (V)...............................................................................14 -

Marika Day's Love Your Gut Month
Week 3: Probiotics and prebiotics OneLife by AIA Vitality Marika Day’s Love Your Gut Month Week 3: Probiotics and prebiotics OneLife by AIA Vitality In the third instalment of Love Your Gut Month, we’re looking at probiotics and prebiotics. Don’t let their similar names fool you: probiotics and prebiotics play very different roles in your body. And both are important for maintaining a healthy gut! So, what’s the difference? Spot the difference OneLife by AIA Vitality Probiotics Effects on your health Bacteria are everywhere. Some of them are good while First up, probiotics fight bad bacteria. They’re your others – well, not so much. inner-most allies, reducing inflammation, increasing immune function, helping you maintain a healthy weight Probiotics are the good kind. They are healthy gut flora and even improving your mood. of various species found in live-culture and fermented products like yoghurt and kombucha. Good bacteria thrive in your gut. Having a balanced diet including both probiotics and prebiotics is essential to keeping them – and therefore you – healthy. Prebiotics Prebiotics are fibres from food that feed the probiotics in your gut. Think of them as fuel for the probiotics – bacteria Glossary gotta eat, too. Live culture: means the bacteria in fermented Prebiotics aren’t advertised at us the way probiotics are, food is still living, and has not been destroyed in the because, well, they’re everywhere. Think onion. Think production process. garlic. Think nuts. Fermented: food preserved by a chemical reaction between the food’s natural sugars and bacteria. What about antibiotics? Am I eating enough? Antibiotics are designed to kill bacteria, and, unfortunately, There’s no tell-tale sign that you’re getting enough don’t distinguish between good and bad. -

(12) United States Patent (10) Patent No.: US 8,268,014 B2 Fröhling (45) Date of Patent: *Sep
USOO8268O14B2 (12) United States Patent (10) Patent No.: US 8,268,014 B2 Fröhling (45) Date of Patent: *Sep. 18, 2012 (54) HAIR DYEING COMPOSITION compounds of formulae (2); (3) and (4): wherein D is the radical of a diazo component of the formula (2a); (2b); (2c); (75) Inventor: Beate Fröhling, Neustadt (DE) (2d); (2f); (2g) or (2h). The invention also relates to the dyeing methods for dyeing keratinous fibers comprising Such dyeing (73) Assignee: BASFSE Ludwigshafen (DE) composition. (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 (1) U.S.C. 154(b) by 0 days. R6 2 Rs This patent is Subject to a terminal dis- R O R4 claimer. NN O nN.1 (21) Appl. No.: 13/148,685 R k 2 (22) PCT Filed: Feb. 19, 2010 -- (2) D-N (86). PCT No.: PCT/EP2010/052097 Y-k S371 (c)(1), (3) (2), (4) Date: Sep. 30, 2011 D-N-K" (87) PCT Pub. No.: WO2010/097338 H 4. PCT Pub. Date: Sep. 2, 2010 R11 (4) (65) Prior Publication Data / \ R-N+ \ R13 US 2012/OO17931 A1 Jan. 26, 2012 o N-y (30) Foreign Application Priority Data / S1 R15 W Feb. 25, 2009 (EP) ..................................... 09 153589 V (51) Int. Cl. R14 A61O 5/10 (2006.01) (2a) CO7D 265/00 (2006.01) X (52) U.S. Cl. ............. 8/405; 8/407; 8/409; 8/426; 8/466; : l 8/565; 8/567; 8/568; 8/570; 8/572; 8/574; le 544/103 Rs (58) Field of Classification Search ............. -

SIGHI Food Compatibility List
Swiss Interest Group Histamine Intolerance (SIGHI) www.mastzellaktivierung.info | www.histaminintoleranz.ch Food Compatibility List Sort order: alphabetic, with categories. Updated: 2016-04-01 Compatibility list for diagnostic and thera- The specifications apply only for the pure foods peutic elimination diet at histaminosis (mast with no additives! For example, it applies only for cell activity syndrome MCAS, mastocytosis, pure natural cream, but not for cream with addi- tives. Additives are sometimes also hidden in sta- histamine intolerance), compiled from vari- ple foods where you would not expect them. ous sources and based on experience reports Therefore, please always read the list of ingredi- ents on the packaging. Compatibility scale Lactose Mechanisms affecting histamine metabolism 0 Free from lactose The list serves as a rough guide for the assess- 1 Low lactose content or may sometimes contain ment of the histamine potential, i.e. for the dose- lactose depending on the recipe dependent and partly individually different com- 2 Medium lactose content. Try out acceptable amount. patibility, which is influenced by various mecha- 3 High lactose content nisms. The reason for the incompatibility is spec- - No general statement possible ified in the list with the following letters: ? Insufficient or contradictory information H!: Highly perishable, rapid formation of Gluten histamine! 0 Gluten-free H: High histamine content 1 May contain gluten 3 Contains gluten A: Other biogenic amines - No general statement possible L : Liberators of mast cell mediators ? Insufficient or contradictory information (=histamine liberators) B: Blocker (=inhibitors) of diamine oxidase Histamine or of other histamine degrading enzymes 0 Well tolerated, no symptoms expected at usual in- take 1 Moderately compatible, minor symptoms, occa- sional consumption of small quantities is often tol- Not all foods are equally intolerable for all con- erated cerned, depending on the individual physical 2 Incompatible, significant symptoms at usual intake causes of histaminosis. -

Tulsa Vegan Guide
Tulsa Vegan Guide Photo courtesy of Oliver and Friends Farm Rescue and Sanctuary in Luther, Oklahoma. Check out their facebook to donate/volunteer. The Tulsa Guide for eating out/shopping compassionately and environmentally-friendly. (Check out the Tulsa Vegan Guide Facebook Group or email [email protected] for updates) 1 TVG The Tulsa Vegan Guide A Guide in Progress Here you will find a few detailed lists of some different restaurants in the Tulsa area that provide vegan-friendly dishes as well as product alternatives found in our city. I am also subject to human error, if you find anything incorrect in this guide, please contact me via the Facebook Group or [email protected] CAUTION: These are not allergen menus. Make sure to communicate with the various restaurants on any cross- contamination issues. For more support: -Tulsa Vegan Guide’s Facebook Group for updates. -Tulsa Vegan Network (Facebook Group) -New Vegan Support (Facebook Group) -Vegan Amino (Phone App) It’s like a vegan Facebook! -www.Challenge22.com (22 Day Vegan Challenge Website for a free nutritionist) “Our unwillingness to see personhood in nonhuman animals does not diminish their personhood; it diminishes our humanity.”- The Abolitionist Vegan Society 2 Table of Contents Highlights……………………………………………………………………………….….….4 Drive Thrus…………………………………………………………………….……….……..6 Sandwiches/Subs………………..…………………………………………..…………...12 Mexican Food…………………………………………………………………………..……20 Asian Food………………………………………………………………………………….….27 Pizza/Italian Food…………………………………………………….……………….……41 -
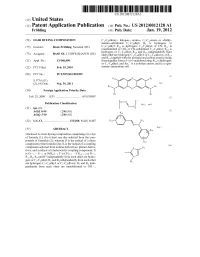
DC ICC R4 An: (30) Foreign Application Priority Data S-S-S-N- R2 R3 Feb
US 201200 12128A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0012128 A1 Fröhling (43) Pub. Date: Jan. 19, 2012 (54) HAIR DYEING COMPOSITION C-Calkoxy-, halogen-, amino-, C-C-mono or -dialky lamino-Substituted C-C alkyl; Rs is hydrogen; or (75) Inventor: Beate Fröhling, Neustadt (DE) C-C alkyl; R is hydrogen; C-C alkyl, or CN; R is unsubstituted or OH- or CN-substituted C-C alkyl; R is hydrogen; or C-C alkyl; R and R independently from (73) Assignee: BASFSE, LUDWIGSHAFEN (DE) each other are hydrogen; C-C alkyl; or C-C alkoxy, or Rs and R together with the nitrogen and carbon atoms joining (21) Appl. No.: 13/148,690 them togetherform a 5- or 6-membered ring; Rs is hydrogen; or C-C alkyl, and An is a colorless anion; and (c) a qua (22) PCT Filed: Feb. 19, 2010 ternary ammonium salt. (86). PCT No.: PCT/EP2010/052099 (1) R6 N Rs S371 (c)(1), 21 (2), (4) Date: Sep. 30, 2011 R DC ICC R4 An: (30) Foreign Application Priority Data S-S-S-N- R2 R3 Feb. 25, 2009 (EP) ................................... 09 1535807 (2) D-N -- Publication Classification V An; and (51) Int. Cl. N-K A6 IK 8/49 (2006.01) (3) A61O 5/10 (2006.01) / f (52) U.S. Cl. .................................. 132/208; 8/426; 8/.407 R-/ \ \ Ji? o N-N An: (57) ABSTRACT VR 14 Disclosed is a hair dyeing composition comprising (a) a dye of formula (1); (b) at least one dye selected from the com () pounds of formulae (2); wherein D is the radical of a diazo R13 component of the formula (2a); K is the radical of a coupling (2a) component selected from aniline derivatives; phenol deriva R7: tives; and a radical of a heterocyclic coupling component; X is O—; —S ; or N(Rs)—;Y is CH=; —CRs—; or N=: R.