The 21St Century Cerebellum: an Evolution of Cognitive Functions, Connections, Disorders, and Pharmacotherapeutic Modulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of Cerebellum Rajasekhar Sajja Srinivasa Siva Naga
Chapter Anatomy of Cerebellum Rajasekhar Sajja Srinivasa Siva Naga Abstract The cerebellum receives inputs from spinal cord, cerebrum, brainstem, and sensory systems of the body and controls the motor system of the body. The Cerebellum harmonizes the voluntary motor activities such as maintenance of posture and equilibrium, and coordination of voluntary muscular activity including learning of the motor behaviours. Cerebellum occupies posterior cranial fossa, and it is relatively a small part of the brain. It weighs about one tenth of the total brain. Cerebellar lesions do not cause motor or cognitive impairment. However, they cause slowing of movements, tremors, lack of equilibrium/balance. Complex motor action becomes shaky and faltering. Keywords: Cerebellum, Spinocerebellar ataxia, Cortex, Medulla, Peduncles, Nuclei 1. Introduction The Cerebellum is the largest part of the hindbrain and develops from the alar plates (rhombic lips) of the metencephalon. It lies between the temporal and occipital lobes of cerebrum and the brainstem in the posterior cranial fossa. It is attached to the posterior surface of the brainstem by three large white fibre bundles. It is attached to the midbrain by superior cerebel- lar peduncle, pons by middle cerebellar peduncle, and medulla by inferior cerebellar peduncle. Cerebellum is concerned with three primary functions: a) coordination of voluntary motor functions of the body initiated by the cerebral cortex at an uncon- scious level, b) maintenance of balance, and posture, c) Maintenance of muscle tone. It receives and integrates the sensory inputs from the cerebrum and the spinal cord necessary for a planning and smooth coordination of the movements [1]. Cerebellar lesions result in irregular and uncoordinated, awkward intentional muscle movements. -

A Model of Long-Term Memory Storage in the Cerebellar Cortex: a Possible Role for Plasticity at Parallel Fiber Synapses Onto Stellate͞basket Interneurons
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14200–14205, December 1997 Psychology A model of long-term memory storage in the cerebellar cortex: A possible role for plasticity at parallel fiber synapses onto stellateybasket interneurons GARRETT T. KENYON† Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, 6431 Fannin, Houston, TX 77030 Edited by Richard F. Thompson, University of Southern California, Los Angeles, CA, and approved October 3, 1997 (received for review April 14, 1997) ABSTRACT By evoking changes in climbing fiber activity, Despite evidence supporting their postulated role in motor movement errors are thought to modify synapses from parallel learning, theoretical arguments suggest that memories stored fibers onto Purkinje cells (pf*Pkj) so as to improve subse- at pf*Pkj synapses would be susceptible to long-term degra- quent motor performance. Theoretical arguments suggest dation as a result of ongoing motor adaptation. First, motor there is an intrinsic tradeoff, however, between motor adap- memories are likely to be distributed across overlapping sets of tation and long-term storage. Assuming a baseline rate of pf*Pkj synapses. If motor memories did not overlap, the motor errors is always present, then repeated performance of storage capacity of pf*Pkj synapses would be greatly reduced any learned movement will generate a series of climbing (12, 17). Second, it is reasonable to assume that the pattern of fiber-mediated corrections. By reshuffling the synaptic synaptic weights necessary to effect any given movement is weights responsible for any given movement, such corrections non-unique. If the activity of a Purkinje cell depends only on will degrade the memories for other learned movements stored the total sum of the synaptic input at any given moment, then in overlapping sets of synapses. -

Anatomy of Cerebellum and Relevant Connections
Anatomy of Cerebellum and Relevant Connections Lecture (14) . Important . Doctors Notes Please check our Editing File . Notes/Extra explanation هذا العمل مبني بشكل أساسي على عمل دفعة 436 مع المراجعة {ومنْْيتو َ ّكْْع َلْْا ِّْللْفَهُوْْحس بهْ} َ َ َ َ َ َ َ َ َ ُ ُ والتدقيق وإضافة المﻻحظات وﻻ يغني عن المصدر اﻷساسي للمذاكرة . Objectives At the end of the lecture, students should be able to: Describe the External features of the cerebellum (lobes, fissures). Describe briefly the Internal structure of the cerebellum. List the name of Cerebellar Nuclei. Relate the Anatomical to the Functional Subdivisions of the cerebellum. Describe the Important connections of each subdivision. Describe briefly the Main Effects in case of lesion of the cerebellum. Cerebellum o Origin: from Hindbrain. Playlist o Position: lies behind Pons & Medulla Separated from them by Fourth ventricle. o Connection: to the brainstem by Inferior, Middle & Superior Cerebellar Peduncles. (medulla) (pons) (midbrain) Extra Cerebellum has 3 fissures: - 2 main (primary) fissures (related to lobes): primary and secondary Cerebellum (posterolateral) - Horizontal fissure (largest/deepest) External Features and not related to lobes o Superior It consists of two Cerebellar Hemispheres joined vermis in midline by the Vermis. and paravermis (intermediate zone) is between vermis and hemisphere inferior o Its surface is highly convoluted forming Folia vermis (like gyri), separated by Fissures (like sulci). Anatomical Subdivision 1. Anterior lobe: in front of primary fissure, on the superior surface. 2. Posterior (middle) lobe: behind primary fissure (Between Primary & Secondary/posterolateral fissures). 3. Flocculonodular lobe: in front of secondary (Posterolateral) fissure, on the inferior surface . -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

The Stellate Cells of the Rat's Cerebellar Cortex*
Z. Anat. Entwickl.-Gesch. 136, 224--248 (1972) by Springer-Verlag 1972 The Stellate Cells of the Rat's Cerebellar Cortex* Victoria Chan-Palay and Sanford L. Palay Departments of Neurobiology and Anatomy, Harvard Medical School, Boston, Massachusetts Received February 18, 1972 Summary. Stellate cells were studied in rapid Golgi preparations and in electron micro- graphs. These small neurons can be classified on the basis of their position in the molecular layer and the patterns of their dendritic and axonal arborizations as follows: (1) superficial cells with short, contorted dendrites and a circumscribed axonal arbor (upper third of the molecular layer) ; (2) deep stellate cells with radiating, twisted dendrites and with long axons giving rise to thin, varicose collaterals (middle third of the molecular layer); (3) deep stellate cells with similar dendrites and long axons giving collaterals to the basket around the Purkinje cell bodies (middle third of the molecular layer). An important characteristic of the stellate cell axon is that it generates most of its collaterals close to its origin. Even in long axon cells, only a few collaterals issue from the more distant parts of the axon. These forms contrast with the basket cell, which sends out long, straighter dendrites, and an extended axon that first emits branches at some distance from its origin. Furthermore, basket cell axon collaterals are usually stout in contrast to the frail, beaded collaterals of the stellate cell axon. The two cell types are considered to be distinct. In electron micrographs stellate cells display folded nuclei and sparse cytoplasm with the characteristics usual for small neurons. -

14-Anatomy of the Cerebellum and the Relevant Connections
Dr. Ahmed Fathalla Ibrahim Professor of Anatomy OBJECTIVES At the end of the lecture, students should: qDescribe the External features of the cerebellum (lobes, fissures). qDescribe briefly the Internal structure of the cerebellum. qList the name of Cerebellar Nuclei. qRelate the Anatomical to the Functional Subdivisions of the cerebellum. qDescribe the Important connections of each subdivision. qDescribe briefly the Main Effects in case of lesion of the cerebellum. • ORIGIN : CEREBELLUM • From Hindbrain. • Position : • lies behind Pons & Medulla Separated from them by Fourth ventricle. THE CEREBELLUM qCONNECTION TO BRAIN STEM: qby Inferior, Middle & Superior Cerebellar Peduncles. EXTERNAL FEATURES qIt consists of two Cerebellar Hemispheres joined in midline by the Vermis. qIts surface is highly convoluted forming Folia, separated by Fissures. ANATOMICAL SUBDIVISION Primary Fissure Superior Surface Posterolateral = Secondary Fissure Anterroinferior Surface 1. Anterior lobe: in front of primary fissure, on the superior surface. 2. Posterior (middle) lobe: behind primary fissure (Between Primary & Secondary fissures = posterolateral). 3. Flocculonodular lobe: in front of secondary (Posterolateral) fissure, on the inferior surface . ANATOMICAL SUBDIVISION CONSTITUENTS (Internal Structure and Nuclei of Cerebellum) 1. Outer grey matter: cerebellar cortex. 2. Inner white matter: cerebellar medulla. 3. Deeply seated nuclei in white matter: from medial to lateral: • Fastigial nucleus. • Globose nucleus. • Emboliform nucleus. • Dentate nucleus: largest one. CEREBELLAR CORTEX q Divided into 3 layers: 1. Outer molecular layer 2. Intermediate Purkinje cell layer 3. Inner granular layer CEREBELLAR MEDULLA AFFERENT FIBRES: q Climbing fibres: from inferior olivary nucleus, relay to purkinje cells q Mossy fibres: rest of fibres: 1. From vestibular nuclei 2. From spinal cord 3. From pons • They relay to granule cells which in turn relay to purkinje cells. -
Homo Sapiens (445) MEDIUM (=IQR) HIGH
Dataset: 445 anatomical parts from data selection: HS_mRNASeq_HUMAN_GL-1 Showing 2 measure(s) of 2 gene(s) on selection: HS-0 TMPRSS2 ACE2 Level of expression Homo sapiens (445) MEDIUM (=IQR) HIGH 0 1 2 3 4 5 6 7 8 9 10 11 samples avg. expr. Tissue 3435 1.42 gestational structure 4 1.92 extraembryonic tissue / fluid 4 1.92 placenta 4 1.92 alimentary system 721 3.93 gastrointestinal tract 519 4.83 mouth (oral cavity) 7 0.87 oral mucosa (unspecified) 4 1.13 salivary gland 3 0.54 stomach 34 2.82 gastric (stomach) region 31 2.90 stomach cardia 11 2.13 stomach body 11 4.06 pyloric antrum 9 2.42 intestine 463 5.14 large intestine 294 3.48 caecum (cecum) 7 3.17 apex of caecum (appendix) 3 1.96 colorectum 287 3.49 colon 207 3.60 colonic mucosa 84 3.43 proximal colon 35 3.20 proximal colonic mucosa 35 3.20 ascending colon 30 3.32 transverse colon 5 2.53 distal colon 31 2.94 distal colonic mucosa 31 2.94 descending colon 6 2.51 sigmoid colon 25 3.05 rectosigmoid colon 5 2.33 rectosigmoid junction 6 3.47 rectum 62 3.00 small intestine 168 8.07 ileum 157 8.36 ileal mucosa 76 8.78 esophagus (oesophagus) 15 1.60 liver and biliary system 130 1.50 liver 119 1.36 gall bladder 3 6.94 bile duct 8 1.56 intrahepatic bile duct 8 1.56 pancreas 72 1.83 pancreatic islet (islet of Langerhans) 70 1.87 circulatory system 791 0.21 cardiovascular system 26 3.51 heart 19 3.39 heart muscle (myocardium, cardiac muscle) 7 4.10 heart ventricle 3 3.88 heart left ventricle 3 3.88 left ventricle free wall 3 3.88 vascular system 7 3.83 blood vessel 7 3.83 artery 7 3.83 coronary -

The Developmental Genetics of the Cerebellum and the Genetic Bases of Known Cerebellar Disorders
THE DEVELOPMENTAL GENETICS OF THE CEREBELLUM AND THE GENETIC BASES OF KNOWN CEREBELLAR DISORDERS A Review of Developmental Genetics of the Cerebellum and the Genetic Bases of known Cerebellar Disorders Olakada Favour Adamba 17/MHS01/254 Department of Medicine and Health Sciences College of Medicine and Health Sciences Afe Babalola University ANA 303: Neuroanatomy July, 2020 The Developmental Genetics of Cerebellum and the Genetic Bases of known Cerebellar Disorders: A Literature Review Olakada Favour Adamba1 Abstract The internal structure of the cerebellum is an intriguing paradox; its cytoarchitecture is relatively simple compared to the connections between its neurons, which are wired into a complex array of gene expression domains and functional circuits. The genetic research of cerebellar development has provided a great deal of information about the molecular events directing the formation of the cerebellum. The developmental mechanisms that coordinate the establishment of cerebellar structure and circuitry provide a powerful model for understanding how a functional brain develops and its significance in cerebellar disorders and diseases. The cellular makeup of the cerebellum is derived from two primary germinal matrices (the ventricular zone and a specialized germinal matrix called the rhombic lip). Each matrix/zone expresses a specific set of genes that establish the cell lineages within the cerebellar anlage. Then, cohorts of differentiated projection neurons and interneuron progenitors migrate into the developing cerebellum. thereafter, a number of remarkable patterning events occur. Altogether, structural and molecular organisations are thought to support the proper connectivity between incoming afferent projections and their target cells. Key words: Cerebellum, circuitry, genetic, development, disorders. I. Introduction The cerebellum (‘little brain’) resides at the anterior end of the hindbrain and is classically defined by its role in sensory-motor processing (Buckner, 2013). -

Nervous System
Nervous system 1 CELLS IN CNS Ectodermal origin • Astrocytes Oligodendrocytes • Astrocytes: • blood brain barrier • 2 types • Fibrous astrocytes in white matter • Protoplasmic astrocytes in gray matter • Oligodendrocytes • Form myelin in CNS 2 Mesodermal origin Microglia • do not develop from cells of neural tube • They come from bone marrow. • These are called microglia • They resemble tissue macrophages. 3 NEURON 4 • Nerve has axon and dendrites and a cell body • Axon starts from axon hillock and ends in terminal buttons which contains vesicles with neurotransmitters. • Nodes of Ranvier: 1 micro metre constrictions, 1mm apart. • Nerve surrounded by myelin sheath . • Myelin formed by schwann cells in PNS but in CNS it is formed by Oligodendrogliocytes. • Transmisssion of transmitters by axoplasmic flow. 5 • Electric Potentials • Non Propagative Propagative. • ALL OR NONE LAW: If the threshold is attained, no change in action potential even if intensity of stimulus is increased. • Refractory Period • Absolute Relative • From firing level to 1/3 from this pt. to start of • Repolarisation after depolarisation. 6 • Myelinated fibres conduct faster • SALTATORY CONDUCTION: Jumping from one node to the other • Depolarisation due to entry of Na ions • Repolarisation due to efflux of K ions • Action potentials starts in axon hillock due to increased Na channels • Energy source is Na K ATP ase. 7 • In CNS neurons are surrounded by glial cells. • 3 types of neuroglia • 1) Microglia - Scavenger cells • 2)Oligodendrocytes :- Forms myelin • 3)Astrocytes: 2 types, Forms blood brain barrier • A) Fibrous • B) Protoplasmic 8 AP reaches pre synaptic terminal Ca Channels open Ca influx Fusion of vesicles with plasma memb Exocytosis 9 Release of neurotransmitter Endocytosis Endosome formation Budding of vesicles from endosome 10 PRESYNAPTIC INHIBITION: Axo axonal synapse Activation of Opening of Direct Presynaptic K channels inhibition Receptors Inc.Cl entry Inc. -
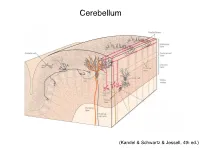
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

The Modifiable Neuronal Network of the Cerebellum
Japanese Journal of Physiology, 34, 781-792, 1984 MINIREVIEW The Modifiable Neuronal Network of the Cerebellum M asao ITO Department of Physiology, Faculty of Medicine, University of Tokyo, Bunkyo-ku, Tokyo, 113 Japan An outstanding feature of the cerebellum known since the classic histological work of Ramon y Cajal is the relative simplicity and highly ordered geometry of its neuronal network. The cerebellar neuronal network consists of five major types of cortical neurons (Purkinje, basket, stellate, Golgi, and granule cells), two major types of afferents (mossy and climbing fibers), and the subcortical cells in the vestibular and cerebellar nuclei. When the neuronal network structure of the cerebellum was comprehensively dissected and described in great detail in the 1960s, and summarized by ECCLES,ITO, and SZ.ENTAGOTHAI[8], it was taken as a challenge to understand more completely the complex functions of the central nervous system using these fundamental studies of the brain as groundwork for future elucidation. As the gap between our understanding of the brain and our knowledge of neuronal networks is in general so large, hope has arisen that cere- bellar physiology may successfully fill the gap rather soon. Rigorous efforts using numerous newly-introduced techniques have been devoted during the past two decades, yielding remarkable progress that meets expectations at least to some extent. A number of problems remain to be solved, however, before we fully understand the cerebellum. It seems important at this point to review the major achievements of the past two decades in order to gain a clearer perspective of cerebellar physiology for the coming decade. -

Anatomy of Cerebellum & Relevant Connections
Anatomy of Cerebellum & Relevant Connections Neuroanatomy block-Anatomy-Lecture 14 Editing file Objectives At the end of the lecture, students should be able to: ● Describe the external features of the cerebellum (lobes, fissures). ● Describe briefly the internal structure of the cerebellum. ● List the name of cerebellar nuclei. ● Relate the anatomical to the functional subdivisions of the cerebellum. ● Describe the important connections of each subdivision. ● Describe briefly the main effects in case of lesion of the cerebellum. Color guide ● Only in boys slides in Green ● Only in girls slides in Purple ● important in Red ● Notes in Grey The Cerebellum Origin : from hindbrain,lies behind Pons & Medulla Separated from them by Fourth ventricle. Connection To Brain Stem : by inferior, middle & superior cerebellar peduncles. External Features: It consists of two cerebellar hemispheres joined in midline by the vermis Its surface is highly convoluted forming folia separated by fissures. Anatomical Subdivision Posterior (middle) lobe: Flocculonodular lobe : Anterior lobe: in front behind primary fissure in front of secondary of primary fissure on (Between Primary & (Posterolateral) the superior surface. Secondary fissures fissure on the inferior = posterolateral) surface . 3 Constituents Internal Structure and Nuclei of Cerebellum Inner white matter: cerebellar medulla. Afferent Fibres: (4 afferent fibers) Deeply seated nuclei in ● Outer grey matter: Climbing fibres: (direct to purkinje cells). from inferior olivary nucleus, relay to purkinje cells white matter: ● cerebellar cortex Mossy fibres: (indirect to purkinje cells) rest of fibres: from medial to lateral: Divided into 3 layers: 1. From vestibular nuclei 2. From spinal cord 3. From pons 1. Fastigial: smallest one 1. Outer molecular layer They relay to granule cells which in turn relay to purkinje cells and most medial Finally all afferent fibres passing through the medulla relay to purkinje cells in the cortex.