Tranquilizers and Anesthesia 1966
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Pharmacology of Autonomic Failure: from Hypotension to Hypertension
1521-0081/69/1/53–62$25.00 http://dx.doi.org/10.1124/pr.115.012161 PHARMACOLOGICAL REVIEWS Pharmacol Rev 69:53–62, January 2017 Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics ASSOCIATE EDITOR: STEPHANIE W. WATTS The Pharmacology of Autonomic Failure: From Hypotension to Hypertension Italo Biaggioni Division of Clinical Pharmacology, Departments of Medicine and Pharmacology, Vanderbilt University, Nashville, Tennessee Abstract .....................................................................................53 I. Introduction . ...............................................................................54 A. Overview of Normal Cardiovascular Autonomic Regulation ...............................54 B. The Baroreflex . .........................................................................54 C. Pathophysiology of Orthostatic Hypotension and Autonomic Failure. ....................54 D. Ganglionic Blockade as a Pharmacological Probe To Understand Autonomic Failure.......55 E. Autonomic Failure as a Model To Understand Pathophysiology . ..........................55 II. Targeting Venous Compliance in the Treatment of Orthostatic Hypotension...................55 III. Pharmacology of Volume Expansion. .......................................................56 Downloaded from A. Fludrocortisone . .........................................................................56 B. Erythropoietin . .........................................................................56 IV. Replacing Noradrenergic Stimulation -

University Microfilms
INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You w ill find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Wo 2010/075090 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 1 July 2010 (01.07.2010) WO 2010/075090 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 409/14 (2006.01) A61K 31/7028 (2006.01) kind of national protection available): AE, AG, AL, AM, C07D 409/12 (2006.01) A61P 11/06 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2009/068073 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 December 2009 (15.12.2009) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, (26) Publication Language: English TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/122,478 15 December 2008 (15.12.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): AUS- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, PEX PHARMACEUTICALS, INC. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Appendices Feb 2010 Inclusion of Cobalt and 2019 Fei Prohibited
APPENDIX 2 CLASSIFICATION OF PROHIBITED SUBSTANCES 1. This classification describes the types of Prohibited Substances and places same in specific categories. The list shall be published on the Club’s Notice Board. CLASS I Drugs that have the greatest pharmacological potential to affect the racing performance of a horse and which have no accepted medical use in a racehorse. These include substances which are potent stimulants of the Central Nervous System (CNS). Examples of drugs in this Class include, but are not limited to: Amphetamines, fentanyl, etorphine, cocaine, morphine, meperidine, opiates, opium derivatives, synthetic opiods, psychoactive drugs. CLASS II Drugs that have a high pharmacological potential for affecting the racing performance of a horse. These include: (i) substances which are pharmacologically active in altering the cons (ii) substances which are not generally accepted as therapeutic agents in the racing horse; and (iii) substances, some of which may have legitimate use in equine medicine but should not be found in the racing horse, such as injectable local anaesthetics. 1 Groups of drugs in this Class include, but are not limited to: (a) Opiate partial agonists, or agonistsantagonists; (b) Non-opiate psychotropic drugs, which may have stimulant, depressant, analgesic or neuroleptic effects; (c) Miscellaneous drugs which might have a stimulant effect on the CNS; (d) Drugs with prominent CNS depressant action; (e) Antidepressant and antipsychotic drugs, with or without prominent CNS stimulatory or depressant effects; (f) Muscle blocking drugs which have direct neuromuscular blocking action; (g) Local anaesthetics which have a reasonable potential for use as nerve blocking agents (except procaine); and (h) Snake venom and other biologic substances which may be used as nerve blocking agents. -

Ethiopian Food and Drug Authority Annexs to Cosmetics Import, Export and Wholesale Control Directive No. 48/2020
Ethiopian Food and Drug Authority Annexs to Cosmetics Import, Export and Wholesale Control Directive No. 48/2020 March, 2020 Addis Ababa, Ethiopia 1 Annexes to the Cosmetics Import, Export and Wholesale Control Directive No. 48/2020 1) Annex I: Illustrative list of cosmetics by catagories 2) Annex II: List of prohibited substances 3) Annex III: List of substances which cosmetic must not contain except the restrictions laid down 4) Annex IV: List of colorants allowed in cosmetics 5) Annex V: List of preservatives allowed in cosmetics 2 Annex I ILLUSTRATIVE LIST OF COSMETICS BY CATEGORIES Creams, emulsions, lotions, gels and oils for the skin (hands, face, feet, etc.) Face masks (with the exception of chemical peeling products) Tinted bases (liquids, pastes, powders) Make-up powders, after-bath powders, hygienic powders, etc. Toilet soaps, deodorant soaps, etc. Perfumes, toilet waters and eau de cologne Bath and shower preparations (salts, foams, oils, gels, etc.) Depilatories Deodorants and anti-perspirants Hair care products hair tints and bleaches products for waving, straightening and fixing setting products o cleansing products (lotions, powders, shampoos) conditioning products (lotions, creams, oils) hairdressing products (lotions, lacquers, brilliantines) Shaving products (creams, foams, lotions, etc.) Products for making-up and removing make-up from the face and the eyes Products intended for application to the lips Products for care of the teeth and the mouth Products for nail care and make-up Products for -

Nicotinic Ach Receptors
Nicotinic ACh Receptors Susan Wonnacott and Jacques Barik Department of Biology and Biochemistry, University of Bath, Bath BA2 7AY, UK Susan Wonnacott is Professor of Neuroscience in the Department of Biology and Biochemistry at the University of Bath. Her research focuses on understanding the roles of nicotinic acetylcholine receptors in the mammalian brain and the molecular and cellular events initiated by acute and chronic nicotinic receptor stimulation. Jacques Barik was a PhD student in the Bath group and is continuing in addiction research at the Collège de France in Paris. Introduction and there followed detailed studies of the properties The nicotinic acetylcholine receptor (nAChR) is the of nAChRs mediating synaptic transmission at prototype of the cys-loop family of ligand-gated ion these sites. nAChRs at the muscle endplate and in sympathetic ganglia could be distinguished channels (LGIC) that also includes GABAA, GABAC, by their respective preferences for C10 and C6 glycine, 5-HT3 receptors, and invertebrate glutamate-, histamine-, and 5-HT-gated chloride channels.1,2 polymethylene bistrimethylammonium compounds, 7 nAChRs in skeletal muscle have been characterised notably decamethonium and hexamethonium. This DRIVING RESEARCH FURTHER in detail whereas mammalian neuronal nAChRs provided the first evidence that muscle and neuronal DRIVING RESEARCH FURTHER in the central nervous system have more recently nAChRs are structurally different. become the focus of intense research efforts. This In the 1970s, elucidation of the structure and function was fuelled by the realisation that nAChRs in the brain of the muscle nAChR, using biochemical approaches, and spinal cord are potential therapeutic targets for was facilitated by the abundance of nicotinic synapses a range of neurological and psychiatric conditions. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

(19) 11 Patent Number: 6165500
USOO6165500A United States Patent (19) 11 Patent Number: 6,165,500 Cevc (45) Date of Patent: *Dec. 26, 2000 54 PREPARATION FOR THE APPLICATION OF WO 88/07362 10/1988 WIPO. AGENTS IN MINI-DROPLETS OTHER PUBLICATIONS 75 Inventor: Gregor Cevc, Heimstetten, Germany V.M. Knepp et al., “Controlled Drug Release from a Novel Liposomal Delivery System. II. Transdermal Delivery Char 73 Assignee: Idea AG, Munich, Germany acteristics” on Journal of Controlled Release 12(1990) Mar., No. 1, Amsterdam, NL, pp. 25–30. (Exhibit A). * Notice: This patent issued on a continued pros- C.E. Price, “A Review of the Factors Influencing the Pen ecution application filed under 37 CFR etration of Pesticides Through Plant Leaves” on I.C.I. Ltd., 1.53(d), and is subject to the twenty year Plant Protection Division, Jealott's Hill Research Station, patent term provisions of 35 U.S.C. Bracknell, Berkshire RG12 6EY, U.K., pp. 237-252. 154(a)(2). (Exhibit B). K. Karzel and R.K. Liedtke, “Mechanismen Transkutaner This patent is Subject to a terminal dis- Resorption” on Grandlagen/Basics, pp. 1487–1491. (Exhibit claimer. C). Michael Mezei, “Liposomes as a Skin Drug Delivery Sys 21 Appl. No.: 07/844,664 tem” 1985 Elsevier Science Publishers B.V. (Biomedical Division), pp 345-358. (Exhibit E). 22 Filed: Apr. 8, 1992 Adrienn Gesztes and Michael Mazei, “Topical Anesthesia of 30 Foreign Application Priority Data the Skin by Liposome-Encapsulated Tetracaine” on Anesth Analg 1988; 67: pp 1079–81. (Exhibit F). Aug. 24, 1990 DE) Germany ............................... 40 26834 Harish M. Patel, "Liposomes as a Controlled-Release Sys Aug. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -
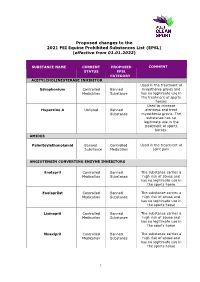
Proposed Changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (Effective from 01.01.2022)
Proposed changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (effective from 01.01.2022) SUBSTANCE NAME CURRENT PROPOSED COMMENT STATUS EPSL CATEGORY ACETYLCHOLINESTERASE INHIBITOR Used in the treatment of Edrophonium Controlled Banned myasthenia gravis and Medication Substance has no legitimate use in the treatment of sports horses Used to increase Huperzine A Unlisted Banned alertness and treat Substance myasthenia gravis. The substance has no legitimate use in the treatment of sports horses. AMIDES Palmitoylethanolamid Banned Controlled Used in the treatment of Substance Medication joint pain ANGIOTENSIN CONVERTING ENZYME INHIBITORS Enalapril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Enalaprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Lisinopril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Moexipril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse 1 Perindoprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse ANTIHISTAMINES Antazoline Controlled Banned The substance has no Medication Substance legitimate use in the sports horse Azatadine Controlled Banned The substance has Medication Substance sedative effects -

Vr Meds Ex01 3B 0825S Coding Manual Supplement Page 1
vr_meds_ex01_3b_0825s Coding Manual Supplement MEDNAME OTHER_CODE ATC_CODE SYSTEM THER_GP PHRM_GP CHEM_GP SODIUM FLUORIDE A12CD01 A01AA01 A A01 A01A A01AA SODIUM MONOFLUOROPHOSPHATE A12CD02 A01AA02 A A01 A01A A01AA HYDROGEN PEROXIDE D08AX01 A01AB02 A A01 A01A A01AB HYDROGEN PEROXIDE S02AA06 A01AB02 A A01 A01A A01AB CHLORHEXIDINE B05CA02 A01AB03 A A01 A01A A01AB CHLORHEXIDINE D08AC02 A01AB03 A A01 A01A A01AB CHLORHEXIDINE D09AA12 A01AB03 A A01 A01A A01AB CHLORHEXIDINE R02AA05 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S01AX09 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S02AA09 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S03AA04 A01AB03 A A01 A01A A01AB AMPHOTERICIN B A07AA07 A01AB04 A A01 A01A A01AB AMPHOTERICIN B G01AA03 A01AB04 A A01 A01A A01AB AMPHOTERICIN B J02AA01 A01AB04 A A01 A01A A01AB POLYNOXYLIN D01AE05 A01AB05 A A01 A01A A01AB OXYQUINOLINE D08AH03 A01AB07 A A01 A01A A01AB OXYQUINOLINE G01AC30 A01AB07 A A01 A01A A01AB OXYQUINOLINE R02AA14 A01AB07 A A01 A01A A01AB NEOMYCIN A07AA01 A01AB08 A A01 A01A A01AB NEOMYCIN B05CA09 A01AB08 A A01 A01A A01AB NEOMYCIN D06AX04 A01AB08 A A01 A01A A01AB NEOMYCIN J01GB05 A01AB08 A A01 A01A A01AB NEOMYCIN R02AB01 A01AB08 A A01 A01A A01AB NEOMYCIN S01AA03 A01AB08 A A01 A01A A01AB NEOMYCIN S02AA07 A01AB08 A A01 A01A A01AB NEOMYCIN S03AA01 A01AB08 A A01 A01A A01AB MICONAZOLE A07AC01 A01AB09 A A01 A01A A01AB MICONAZOLE D01AC02 A01AB09 A A01 A01A A01AB MICONAZOLE G01AF04 A01AB09 A A01 A01A A01AB MICONAZOLE J02AB01 A01AB09 A A01 A01A A01AB MICONAZOLE S02AA13 A01AB09 A A01 A01A A01AB NATAMYCIN A07AA03 A01AB10 A A01