Subtitle 21A-243
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. 2012 AGS Beers Criteria for Potentially
Table 2. 2012 AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Strength of Organ System/ Recommendat Quality of Recomm Therapeutic Category/Drug(s) Rationale ion Evidence endation References Anticholinergics (excludes TCAs) First-generation antihistamines Highly anticholinergic; Avoid Hydroxyzin Strong Agostini 2001 (as single agent or as part of clearance reduced with e and Boustani 2007 combination products) advanced age, and promethazi Guaiana 2010 Brompheniramine tolerance develops ne: high; Han 2001 Carbinoxamine when used as hypnotic; All others: Rudolph 2008 Chlorpheniramine increased risk of moderate Clemastine confusion, dry mouth, Cyproheptadine constipation, and other Dexbrompheniramine anticholinergic Dexchlorpheniramine effects/toxicity. Diphenhydramine (oral) Doxylamine Use of diphenhydramine in Hydroxyzine special situations such Promethazine as acute treatment of Triprolidine severe allergic reaction may be appropriate. Antiparkinson agents Not recommended for Avoid Moderate Strong Rudolph 2008 Benztropine (oral) prevention of Trihexyphenidyl extrapyramidal symptoms with antipsychotics; more effective agents available for treatment of Parkinson disease. Antispasmodics Highly anticholinergic, Avoid Moderate Strong Lechevallier- Belladonna alkaloids uncertain except in Michel 2005 Clidinium-chlordiazepoxide effectiveness. short-term Rudolph 2008 Dicyclomine palliative Hyoscyamine care to Propantheline decrease Scopolamine oral secretions. Antithrombotics Dipyridamole, oral short-acting* May -

Review of Market for Octane Enhancers
May 2000 • NREL/SR-580-28193 Review of Market for Octane Enhancers Final Report J.E. Sinor Consultants, Inc. Niwot, Colorado National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 May 2000 • NREL/SR-580-28193 Review of Market for Octane Enhancers Final Report J.E. Sinor Consultants, Inc. Niwot, Colorado NREL Technical Monitor: K. Ibsen Prepared under Subcontract No. TXE-0-29113-01 National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. Available electronically at http://www.doe.gov/bridge Available for a processing fee to U.S. -

Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances 2017
INTERNATIONAL NARCOTICS CONTROL BOARD Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances 2017 EMBARGO Observe release date: Not to be published or broadcast before Thursday, 1 March 2018, at 1100 hours (CET) UNITED NATIONS CAUTION Reports published by the International Narcotics Control Board in 2017 The Report of the International Narcotics Control Board for 2017 (E/INCB/2017/1) is supplemented by the following reports: Narcotic Drugs: Estimated World Requirements for 2018—Statistics for 2016 (E/INCB/2017/2) Psychotropic Substances: Statistics for 2016—Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 1971 (E/INCB/2017/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances: Report of the International Narcotics Control Board for 2017 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2017/4) The updated lists of substances under international control, comprising narcotic drugs, psychotropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms (“Yellow List”, “Green List” and “Red List”), which are also issued by the Board. Contacting the International Narcotics Control Board The secretariat of the Board may be reached at the following address: Vienna International Centre Room E-1339 P.O. Box 500 1400 Vienna Austria In addition, the following may be used to contact the secretariat: Telephone: (+43-1) 26060 Fax: (+43-1) 26060-5867 or 26060-5868 Email: [email protected] The text of the present report is also available on the website of the Board (www.incb.org). -

Organocatalytic Asymmetric N-Sulfonyl Amide C-N Bond Activation to Access Axially Chiral Biaryl Amino Acids
ARTICLE https://doi.org/10.1038/s41467-020-14799-8 OPEN Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids Guanjie Wang1, Qianqian Shi2, Wanyao Hu1, Tao Chen1, Yingying Guo1, Zhouli Hu1, Minghua Gong1, ✉ ✉ ✉ Jingcheng Guo1, Donghui Wei 2 , Zhenqian Fu 1,3 & Wei Huang1,3 1234567890():,; Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and trans- formations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions. 1 Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. 2 College -
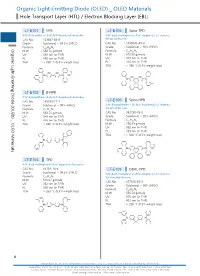
Organic Light-Emitting Diode (OLED) OLED Materials Hole Transport Layer (HTL) / Electron Blocking Layer (EBL)
Organic Light-EmittingOrganic Light-Emitting DiodeHole Transport(OLED) Layer (HTL) / Diode (OLED) _ OLED Materials Electron Blocking Layer (EBL) Organic Light-Emitting Diode (OLED) _ OLED Materials Hole Transport Layer (HTL) / Electron Blocking Layer (EBL) LT-E101 NPB LT-E105 Spiro-TPD N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)-benzidine N,N'-Bis(3-methylphenyl)-N,N'-bis(phenyl)-2,7-diamino- CAS No. : 123847-85-8 9,9-spirobifluorene Grade : Sublimed, > 99.5% (HPLC) CAS No. : 1033035-83-4 : Grade : Sublimed, > 99% (HPLC) Organic Light-Emitting Diode (OLED) _ OLED Materials Formula C44H32N2 M.W. : 588.74 g/mole Formula : C51H38N2 UV : 339 nm (in THF) M.W. : 678.86 g/mole PL : 450 nm (in THF) UV : 379 nm (in THF) TGA : > 350 °C (0.5% weight loss) PL : 416 nm (in THF) TGA : > 280 °C (0.5% weight loss) N N N N LT-E102 β-NPB N,N'-Bis(naphthalen-2-yl)-N,N'-bis(phenyl)-benzidine Spiro-NPB CAS No. : 139255-17-7 LT-E106 Grade : Sublimed, > 99% (HPLC) N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)-2,7-diamino- 9,9-spirobifluorene Formula : C44H32N2 M.W. : 588.74 g/mole CAS No. : 932739-76-9 UV : 349 nm (in THF) Grade : Sublimed, > 99% (HPLC) PL : 416 nm (in THF) Formula : C57H38N2 TGA : > 330 °C (0.5% weight loss) M.W. : 750.93 g/mole UV : 380 nm (in THF) PL : 453 nm (in THF) TGA : > 360 °C (0.5% weight loss) N N N N LT-E103 TPD N,N'-Bis(3-methylphenyl)-N,N'-bis(phenyl)-benzidine CAS No. -

Drugs to Avoid in Patients with Dementia
Detail-Document #240510 -This Detail-Document accompanies the related article published in- PHARMACIST’S LETTER / PRESCRIBER’S LETTER May 2008 ~ Volume 24 ~ Number 240510 Drugs To Avoid in Patients with Dementia Elderly people with dementia often tolerate drugs less favorably than healthy older adults. Reasons include increased sensitivity to certain side effects, difficulty with adhering to drug regimens, and decreased ability to recognize and report adverse events. Elderly adults with dementia are also more prone than healthy older persons to develop drug-induced cognitive impairment.1 Medications with strong anticholinergic (AC) side effects, such as sedating antihistamines, are well- known for causing acute cognitive impairment in people with dementia.1-3 Anticholinergic-like effects, such as urinary retention and dry mouth, have also been identified in drugs not typically associated with major AC side effects (e.g., narcotics, benzodiazepines).3 These drugs are also important causes of acute confusional states. Factors that may determine whether a patient will develop cognitive impairment when exposed to ACs include: 1) total AC load (determined by number of AC drugs and dose of agents utilized), 2) baseline cognitive function, and 3) individual patient pharmacodynamic and pharmacokinetic features (e.g., renal/hepatic function).1 Evidence suggests that impairment of cholinergic transmission plays a key role in the development of Alzheimer’s dementia. Thus, the development of the cholinesterase inhibitors (CIs). When used appropriately, the CIs (donepezil [Aricept], rivastigmine [Exelon], and galantamine [Razadyne, Reminyl in Canada]) may slow the decline of cognitive and functional impairment in people with dementia. In order to achieve maximum therapeutic effect, they ideally should not be used in combination with ACs, agents known to have an opposing mechanism of action.1,2 Roe et al studied AC use in 836 elderly patients.1 Use of ACs was found to be greater in patients with probable dementia than healthy older adults (33% vs. -

Hydroxydopamine Lesions of the Nucleus Accumbens and the Mglur2/3 Agonist LY379268
Neuropsychopharmacology (2003) 28, 1440–1447 & 2003 Nature Publishing Group All rights reserved 0893-133X/03 $25.00 www.neuropsychopharmacology.org Toluene-Induced Locomotor Activity is Blocked by 6- Hydroxydopamine Lesions of the Nucleus Accumbens and the mGluR2/3 Agonist LY379268 1,3 2 ,1 AC Riegel , SF Ali , ED French* 1Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ, USA; 2Neurochemistry Laboratory, Division of Neurotoxicology, National Center for Toxicological Research/US FDA, Jefferson, AR, USA The abuse of volatile inhalants remains a prominent, yet poorly understood, form of substance abuse among youth. Nevertheless, the identification of a mechanism underlying the reinforcing properties of inhalants has been hampered by the lack of a clearly identifiable neural substrate upon which these chemicals act. One ingredient that is common to many abused inhalants is toluene, an organic solvent that is self-administered by nonhuman primates and rodents. Most drugs of abuse have been found to elicit forward locomotion in rats, an effect owing to the activation of mesoaccumbal dopamine (DA) pathways. Thus, the present study was undertaken using two different approaches to determine whether toluene-induced locomotor hyperactivity is also ultimately dependent upon DA neurotransmission in the mesolimbic nucleus accumbens (NAC). Here we report on the effects of 6-hydroxydopamine (6-OHDA) lesions of the NAC or pretreatment with the metabotropic mGlu2/3 receptor agonist LY379268 on toluene-induced locomotor activity. Both procedures, which are known to alter neurotransmission within the NAC, significantly attenuated toluene’s locomotor stimulatory effects. These results provide strong support for a central mechanism of action of inhalants, which in the past has been more typically attributed to general nonspecific mechanisms throughout the brain. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Nomination Background: Ketamine Hydrochloride (CASRN: 1867-66-9)
KETAMINE Nomination TABLE OF CONTENTS Page 1.0 BASIS OF NOMINATION 1 2.0 BACKGROUND INFORMATION 1 3.0 CHEMICAL PROPERTIES 2 3.1 Chemical Identification 2 3.2 Physico-Chemical Properties 3 3.3 Purity and Commercial Availability 4 4.0 PRODUCTION PROCESSES AND ANALYSIS 6 5.0 PRODUCTION AND IMPORT VOLUMES 7 6.0 USES 7 7.0 ENVIRONMENTAL OCCURRENCE 7 8.0 HUMAN EXPOSURE 7 9.0 REGULATORY STATUS 7 10.0 CLINICAL PHARMACOLOGY 7 11.0 TOXICOLOGICAL DATA 13 11.1 General Toxicology 13 11.2 Neurotoxicology 14 12.0 CONCLUSIONS 15 APPENDIX A 17 APPENDIX B 23 APPENDIX C 27 1 KETAMINE 1.0 BASIS OF NOMINATION Ketamine, a noncompetitive NMDA receptor blocker, has been used extensively off - label as a pediatric anesthetic for surgical procedures in infants and toddlers. Recently, Olney and coworkers have demonstrated severe widespread apoptotic degeneration throughout the rapidly developing brain of the 7-day-old rat after ketamine administration. Recent research at FDA has confirmed and extended Olney’s observations. These findings are cause for concern with respect to ketamine use in children. The issue of whether the neurotoxicity found in this animal model (rat) has scientific and regulatory relevance for the pediatric use of ketamine relies heavily upon confirmation of these findings that may be obtained from the conduct of an appropriate study in non-human primates. 2.0 BACKGROUND INFORMATION The issue of potential ketamine neurotoxicity in children surfaced as a result of FDA’s reluctance to approve an NIH pediatric clinical trial using this compound because of its documented neurotoxic effects in young rats (published in several papers over the last ten years by Olney and co-workers). -

The Anticholinergic Toxidrome
Poison HOTLINE Partnership between Iowa Health System and University of Iowa Hospitals and Clinics July 2011 The Anticholinergic Toxidrome A toxidrome is a group of symptoms associated with poisoning by a particular class of agents. One example is the opiate toxidrome, the triad of CNS depression, respiratory depression, and pinpoint pupils, and which usually responds to naloxone. The anticholinergic toxidrome is most frequently associated with overdoses of diphenhydramine, a very common OTC medication. However, many drugs and plants can produce the anticholinergic toxidrome. A partial list includes: tricyclic antidepressants (amitriptyline), older antihistamines (chlorpheniramine), Did you know …… phenothiazines (promethazine) and plants containing the anticholinergic alkaloids atropine, hyoscyamine and scopolamine (Jimson Weed). Each summer, the ISPCC receives approximately 10-20 The mnemonic used to help remember the symptoms and signs of this snake bite calls, some being toxidrome are derived from the Alice in Wonderland story: from poisonous snakes (both Blind as a Bat (mydriasis and inability to focus on near objects) local and exotic). Red as a Beet (flushed skin color) Four poisonous snakes can be Hot as Hades (elevated temperature) found in Iowa: the prairie These patients can sometimes die of agitation-induced hyperthermia. rattlesnake, the massasauga, Dry as a Bone (dry mouth and dry skin) the copperhead, and the Mad as a Hatter (hallucinations and delirium) timber rattlesnake. Each Bowel and bladder lose their tone (urinary retention and constipation) snake has specific territories Heart races on alone (tachycardia) within the state. ISPCC A patient who has ingested only an anticholinergic substance and is not specialists have access to tachycardic argues against a serious anticholinergic overdose. -

3 Drugs That May Cause Delirium Or Problem Behaviors CARD 03 19 12 JUSTIFIED.Pub
Drugs that May Cause Delirium or Problem Behaviors Drugs that May Cause Delirium or Problem Behaviors This reference card lists common and especially problemac drugs that may Ancholinergics—all drugs on this side of the card. May impair cognion cause delirium or contribute to problem behaviors in people with demena. and cause psychosis. Drugs available over‐the‐counter marked with * This does not always mean the drugs should not be used, and not all such drugs are listed. If a paent develops delirium or has new problem Tricyclic Andepressants Bladder Anspasmodics behaviors, a careful review of all medicaons is recommended. Amitriptyline – Elavil Darifenacin – Enablex Be especially mindful of new medicaons. Clomipramine – Anafranil Flavoxate – Urispas Desipramine – Norpramin Anconvulsants Psychiatric Oxybutynin – Ditropan Doxepin – Sinequan Solifenacin – VESIcare All can cause delirium, e.g. All psychiatric medicaons should be Imipramine – Tofranil Tolterodine – Detrol Carbamazepine – Tegretol reviewed as possible causes, as Nortriptyline – Aventyl, Pamelor Gabapenn – Neuronn effects are unpredictable. Trospium – Sanctura Anhistamines / Allergy / Leveracetam – Keppra Notable offenders include: Insomnia / Sleep Valproic acid – Depakote Benzodiazepines e.g. Cough & Cold Medicines *Diphenhydramine – Sominex, ‐Alprazolam – Xanax *Azelasne – Astepro Pain Tylenol‐PM, others ‐Clonazepam – Klonopin *Brompheniramine – Bromax, All opiates can cause delirium if dose *Doxylamine – Unisom, Medi‐Sleep ‐Lorazepam – Avan Bromfed, Lodrane is too high or -

Pharmacological Treatments in Insomnia
Pharmacological treatments in insomnia Sue Wilson Centre for Neuropsychopharmacology, Division of Brain Sciences, Imperial College, London Drugs used in insomnia Licensed for insomnia •GABA-A positive allosteric modulators •melatonin (modified release) •promethazine •diphenhydramine •doxepin (USA) Unlicensed prescribed frequently •antihistamines (and OTC) •antidepressants Sometimes prescribed drugs for psychosis Some GABA-A positive allosteric modulators Drugs acting at the GABA-A benzodiazepine receptor zopiclone zolpidem zaleplon benzodiazepines eg temazepam, lorazepam (safe in overdose, as long as no other drug involved) Drugs acting at the barbiturate/alcohol receptor chloral hydrate/chloral betaine clomethiazole (dangerous in overdose) GABA calms the brain Gamma aminobutyic acid (GABA) is the main inhibitory transmitter in the mammalian central nervous system. It plays the principal role in reducing neuronal excitability and its receptors are prolific throughout the brain, in cortex, limbic system, thalamus and cerebellum sedative Increase anticonvulsant GABA anxiolytic function ataxia, memory effects Effects of GABA-A positive allosteric modulators •These drugs enhance the effect of GABA, the main inhibitory neurotransmitter in the brain •They all produce sedation, sleep promotion, ataxia, muscle relaxation, effects on memory, anticonvulsant effects •Therefore for insomnia the duration of action of the drug is important – these effects are unwanted during the day Effects of these GABA-ergic drugs on sleep EEG/PSG • Appearance of