Iran Wetlands: Development Needs and Conservation Management – Dr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review and Updated Checklist of Freshwater Fishes of Iran: Taxonomy, Distribution and Conservation Status
Iran. J. Ichthyol. (March 2017), 4(Suppl. 1): 1–114 Received: October 18, 2016 © 2017 Iranian Society of Ichthyology Accepted: February 30, 2017 P-ISSN: 2383-1561; E-ISSN: 2383-0964 doi: 10.7508/iji.2017 http://www.ijichthyol.org Review and updated checklist of freshwater fishes of Iran: Taxonomy, distribution and conservation status Hamid Reza ESMAEILI1*, Hamidreza MEHRABAN1, Keivan ABBASI2, Yazdan KEIVANY3, Brian W. COAD4 1Ichthyology and Molecular Systematics Research Laboratory, Zoology Section, Department of Biology, College of Sciences, Shiraz University, Shiraz, Iran 2Inland Waters Aquaculture Research Center. Iranian Fisheries Sciences Research Institute. Agricultural Research, Education and Extension Organization, Bandar Anzali, Iran 3Department of Natural Resources (Fisheries Division), Isfahan University of Technology, Isfahan 84156-83111, Iran 4Canadian Museum of Nature, Ottawa, Ontario, K1P 6P4 Canada *Email: [email protected] Abstract: This checklist aims to reviews and summarize the results of the systematic and zoogeographical research on the Iranian inland ichthyofauna that has been carried out for more than 200 years. Since the work of J.J. Heckel (1846-1849), the number of valid species has increased significantly and the systematic status of many of the species has changed, and reorganization and updating of the published information has become essential. Here we take the opportunity to provide a new and updated checklist of freshwater fishes of Iran based on literature and taxon occurrence data obtained from natural history and new fish collections. This article lists 288 species in 107 genera, 28 families, 22 orders and 3 classes reported from different Iranian basins. However, presence of 23 reported species in Iranian waters needs confirmation by specimens. -
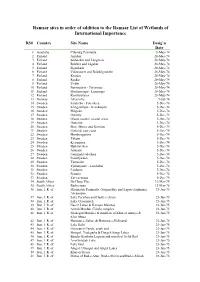
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

LCSH Section K
K., Rupert (Fictitious character) Motion of K stars in line of sight Ka-đai language USE Rupert (Fictitious character : Laporte) Radial velocity of K stars USE Kadai languages K-4 PRR 1361 (Steam locomotive) — Orbits Ka’do Herdé language USE 1361 K4 (Steam locomotive) UF Galactic orbits of K stars USE Herdé language K-9 (Fictitious character) (Not Subd Geog) K stars—Galactic orbits Ka’do Pévé language UF K-Nine (Fictitious character) BT Orbits USE Pévé language K9 (Fictitious character) — Radial velocity Ka Dwo (Asian people) K 37 (Military aircraft) USE K stars—Motion in line of sight USE Kadu (Asian people) USE Junkers K 37 (Military aircraft) — Spectra Ka-Ga-Nga script (May Subd Geog) K 98 k (Rifle) K Street (Sacramento, Calif.) UF Script, Ka-Ga-Nga USE Mauser K98k rifle This heading is not valid for use as a geographic BT Inscriptions, Malayan K.A.L. Flight 007 Incident, 1983 subdivision. Ka-houk (Wash.) USE Korean Air Lines Incident, 1983 BT Streets—California USE Ozette Lake (Wash.) K.A. Lind Honorary Award K-T boundary Ka Iwi National Scenic Shoreline (Hawaii) USE Moderna museets vänners skulpturpris USE Cretaceous-Paleogene boundary UF Ka Iwi Scenic Shoreline Park (Hawaii) K.A. Linds hederspris K-T Extinction Ka Iwi Shoreline (Hawaii) USE Moderna museets vänners skulpturpris USE Cretaceous-Paleogene Extinction BT National parks and reserves—Hawaii K-ABC (Intelligence test) K-T Mass Extinction Ka Iwi Scenic Shoreline Park (Hawaii) USE Kaufman Assessment Battery for Children USE Cretaceous-Paleogene Extinction USE Ka Iwi National Scenic Shoreline (Hawaii) K-B Bridge (Palau) K-TEA (Achievement test) Ka Iwi Shoreline (Hawaii) USE Koro-Babeldaod Bridge (Palau) USE Kaufman Test of Educational Achievement USE Ka Iwi National Scenic Shoreline (Hawaii) K-BIT (Intelligence test) K-theory Ka-ju-ken-bo USE Kaufman Brief Intelligence Test [QA612.33] USE Kajukenbo K. -

Water Dilemma in Isfahan and International Tourists' Effect on It
Water Dilemma in Isfahan and International Tourists’ effect on it By: Sheyma Karimi Supervisor: Saeid Abbasian Master’s dissertation 15 credits Södertörn University | School of Social Science Abstract Tourism is one of the leading industries, in terms of international trading between countries. In addition to receipts received at destinations, international tourism has also generated US$211 billion in exports through international passenger transport services. The study is conducted in Isfahan, a city in center of Iran. The city is unique in its cultural heritage and archeology. It is one of Iran's oldest cities at more than 1,500 years of age. An important cultural and commercial center, Isfahan is Iran's third largest metropolitan area. Isfahan experiences an arid climate, like the rest of the Iranian plateau with low rainfall. Isfahan has a high capacity to attract international tourists to provide a better understanding of Iran’s history, culture, and natural environment to the world. Zayandeh Rud which means “life-giving River” is the largest Iranian plateau and the most important surface water in Isfahan. It starts from Zagros Mountains and ends in the Gavkhouni Swamp, a seasonal salt lake in the southeast of Isfahan. The catchment area has been affected by two drought periods within the last 15 years. Decreasing surface and groundwater availability has been accompanied by an increase in water withdrawal for irrigation, domestic uses, industry, and water transfers to neighboring provinces. This has led to severe ecological and social consequences. This study identifies the potentials of Isfahan in attracting international tourists and also evaluate the water crisis that the city faces. -

Download (Pdf, 5.07
THE HERPETOLOGICAL BULLETIN The Herpetological Bulletin is produced quarterly and publishes, in English, a range of articles concerned with herpetology. These include full-length papers, new methodologies, short communications, natural history notes and book reviews. Emphasis is placed on field studies, conservation, veterinary and behavioural aspects. Authors should read and adhere to the British Ecological Society’s Ethical Policy and Guidelines, a full version of which can be found at https://www.thebhs.org/info-advice/134-bhs-ethics-policy or The Herpetological Bulletin (2017), 141: 46- 18. All submissions are liable to assessment by the editorial board for ethical considerations, and publication may be refused on the recommendation of this committee. Contributors may therefore need to justify killing or the use of other animal procedures, if these have been involved in the execution of the work. Likewise, work that has involved the collection of endangered species or disturbance to their habitat(s) will require full justification. Articles reporting the results of experimental research, descriptions of new taxa, or taxonomic revisions should be submitted to The Herpetological Journal (see inside back cover for Editor’s address). Guidelines for Contributing Authors: 1. See the BHS website for a free download of the Bulletin showing Bulletin style. A template is available from the BHS website www.thebhs.org or on request from the Editor. 2. Contributions should be submitted by email to [email protected]. 3. Articles should be arranged in the following general order: Title Name(s) of authors(s) Address(es) of author(s) (please indicate corresponding author) Abstract (required for all full research articles - should not exceed 10% of total word length) Text acknowledgements References Appendices Footnotes should not be included. -

Mathematical Model for Evaluating of Sediment Transport (Case Study: Karkheh River in Iran)
ISSN (Print) : 0974-6846 Indian Journal of Science and Technology, Vol 8(23), DOI: 10.17485/ijst/2015/v8i23/75246, September 2015 ISSN (Online) : 0974-5645 Mathematical Model for Evaluating of Sediment Transport (Case Study: Karkheh River in Iran) Farhang Azarang1*, Abdol Rasoul Telvari2, Hossein Sedghi1 and Mahmoud Shafai Bajestan3 1Department of Water Science and Engineering, Science and Research Branch, Islamic Azad University, Tehran - 1477893855, Iran; [email protected], [email protected] 2Department of Civil Engineering, Faculty of Engineering, Islamic Azad University, Ahwaz, Iran; [email protected] 3Department of Water Science and Engineering, Shahid Chamran University, Ahwaz, Iran; [email protected] Abstract Reservoir dams are the most important hydraulic structures built on rivers and have a great impact on the river conditions. In this study, MIKE 11 mathematical model in Karkheh river is used in Iran. Karkheh River is one of the most important rivers of Iran on which Karkheh Reservoir Dam is built. MIKE 11 Model is used for simulation of flow and sediment in rivers. The studied Kriavrekr hfelohw R idvoerw annsdtr ceoammp ouft aKtiaornkahle rhe sRueltsse wrveorier cDoammp aarnedd eancdo emvaplausasteesd gweiotmh oebtrsiecr, vhaytdiornaual idc aatan.d C rsoesdsim seecnttios nianlf ogremomateiotrnic oafl cKhaarnkghesh oRfi vKearr kinh Aehb dRoivlkehr aunp asntrde aHmam (Aidbidyeohlk hhyadnr hoymdertormic esttraitci osntast. iMona)n wnienrge’ sc raolcuuglhanteeds sa cnode tfhfiec ibeenst t0 w.0a2y5s wtoa sp rceodniscitd cehreadn gfoesr shape. Longitudinal bed level changes of Karkheh River was estimated from upstream to downstream. Elevation changes of Kwaerrkeh ienhtr roidvuerc ebde. dE nwgaesl uonbdta-Hinaends eant tahned h Aycdkreorms-eWtrhicit est eaqtiuoantsio onfs A obffdeorlekdh abne t(tuerp sptrreedaimct)i oannsd o Hf cahmaindgiehs i(nd othwen Kstarrekahmeh) uRsivinegr MIKE 11 Model. -

Study of the Impact of Form Enclosure in Residential
Science Arena Publications Specialty Journal of Architecture and Construction Available online at www.sciarena.com 2016, Vol 2 (2): 43-52 Study Of The Impact Of Form Enclosure In Residential Complexes On The Sense Of Place Attachment Of Residents (Case study: Shahrakee Ekbatan and Shahrakee Gharb residential complexes)1 Seyedeh Sarah Qodsi1, Jamaluddin Soheili2 1- Department of architecture ,Barajin Science and Research College, Islamic Azad University ,Qazvin, Iran [email protected] (+98) 9124900159 2- Faculty member and assistant professor ,Islamic Azad University ,Qazvin ,Iran (Corresponding) [email protected] (+98) 9123816120 Abstract: The attachment and belonging to the environment in the traditional neighborhood life has always contributed to a sense of responsibility in the residents relative to each other and the city and establishing social partnerships. Today, major changes have occurred in the lifestyle and social communications especially among the neighbors. This has resulted in indifference and separation of the citizens from each other and also from the events that occur within the city and the neighborhood. The increasing process of weakening social relations will eventually lead to social disconnectedness. Since the traditional neighborhoods in Iran enjoyed a built-in enclosure and hierarchy and such enclosure had a significant impact on the residents’ sense of place attachment, this question comes to mind that whether the enclosed form of a residential complex can also affect the level of the inhabitants’ sense of place attachment similar to the traditional neighborhoods? Hence, in this paper we have attempted to examine the lives of the residents of the residential complexes such as Shahrake Ekbatan and Shahrake Gharb in the Greater Tehran to come up with a clear answer to the raised question. -

Consequences of Drying Lake Systems Around the World
Consequences of Drying Lake Systems around the World Prepared for: State of Utah Great Salt Lake Advisory Council Prepared by: AECOM February 15, 2019 Consequences of Drying Lake Systems around the World Table of Contents EXECUTIVE SUMMARY ..................................................................... 5 I. INTRODUCTION ...................................................................... 13 II. CONTEXT ................................................................................. 13 III. APPROACH ............................................................................. 16 IV. CASE STUDIES OF DRYING LAKE SYSTEMS ...................... 17 1. LAKE URMIA ..................................................................................................... 17 a) Overview of Lake Characteristics .................................................................... 18 b) Economic Consequences ............................................................................... 19 c) Social Consequences ..................................................................................... 20 d) Environmental Consequences ........................................................................ 21 e) Relevance to Great Salt Lake ......................................................................... 21 2. ARAL SEA ........................................................................................................ 22 a) Overview of Lake Characteristics .................................................................... 22 b) Economic -

Additional Defensive Behaviours of Dipsas Mikanii (Schlegel, 1837) and Taeniophallus Occipitalis (Jan, 1863) (Serpentes: Dipsadidae)
Herpetology Notes, volume 12: 359-362 (2019) (published online on 0 April 2019) Additional defensive behaviours of Dipsas mikanii (Schlegel, 1837) and Taeniophallus occipitalis (Jan, 1863) (Serpentes: Dipsadidae) Bruno F. Fiorillo1,*, Giordano N. Rossi2, and Marcio Martins3 Snakes evolved defensive behaviours to avoid being 2015). Its diet is specialized in gastropods (Oliveira, detected, injured or killed by predators, and they possess 2001; Marques et al., 2015). an array of such behaviours (see a review in Greene, Taeniophallus occipitalis is a terrestrial, diurnal species 1988). The family Dipsadidae is widespread in the New that is found in leaf-litter (Sawaya et al., 2008; Morato et World and exhibits a high species diversity in Central al., 2011; Marques et al., 2015) of open vegetation types and South America, and the West Indies (Zaher et al., of the Cerrado (Scrocchi and Giraudo, 2005; França 2009; Vidal et al., 2010). Previous studies have reported et al., 2008; Sawaya et al., 2008). It does not seem to different defensive tactics used by several species of this persist in disturbed areas (Sawaya et al., 2008). Its diet family (e.g. Martins and Oliveira, 1998; Martins et al., is composed mainly of anurans and lizards (Yanosky et 2008; Maia-Carneiro et al., 2012; Menezes et al., 2015, al., 1996; Cechin, 1999; Marques et al., 2009). 2017; Atkinson, 2018; Fiorillo et al., 2018). However, Known defensive behaviours of D. mikanii are head there is still much to discover about defensive behavior triangulation, hiding the head, cloacal discharge, and in snakes, and the description and documentation of striking (Marques et al., 2015). -

Origin and Age of Australian Chenopodiaceae
ARTICLE IN PRESS Organisms, Diversity & Evolution 5 (2005) 59–80 www.elsevier.de/ode Origin and age of Australian Chenopodiaceae Gudrun Kadereita,Ã, DietrichGotzek b, Surrey Jacobsc, Helmut Freitagd aInstitut fu¨r Spezielle Botanik und Botanischer Garten, Johannes Gutenberg-Universita¨t Mainz, D-55099 Mainz, Germany bDepartment of Genetics, University of Georgia, Athens, GA 30602, USA cRoyal Botanic Gardens, Sydney, Australia dArbeitsgruppe Systematik und Morphologie der Pflanzen, Universita¨t Kassel, D-34109 Kassel, Germany Received 20 May 2004; accepted 31 July 2004 Abstract We studied the age, origins, and possible routes of colonization of the Australian Chenopodiaceae. Using a previously published rbcL phylogeny of the Amaranthaceae–Chenopodiaceae alliance (Kadereit et al. 2003) and new ITS phylogenies of the Camphorosmeae and Salicornieae, we conclude that Australia has been reached in at least nine independent colonization events: four in the Chenopodioideae, two in the Salicornieae, and one each in the Camphorosmeae, Suaedeae, and Salsoleae. Where feasible, we used molecular clock estimates to date the ages of the respective lineages. The two oldest lineages both belong to the Chenopodioideae (Scleroblitum and Chenopodium sect. Orthosporum/Dysphania) and date to 42.2–26.0 and 16.1–9.9 Mya, respectively. Most lineages (Australian Camphorosmeae, the Halosarcia lineage in the Salicornieae, Sarcocornia, Chenopodium subg. Chenopodium/Rhagodia, and Atriplex) arrived in Australia during the late Miocene to Pliocene when aridification and increasing salinity changed the landscape of many parts of the continent. The Australian Camphorosmeae and Salicornieae diversified rapidly after their arrival. The molecular-clock results clearly reject the hypothesis of an autochthonous stock of Chenopodiaceae dating back to Gondwanan times. -

PARADISE LOST? Developing Solutions to Irans Environmental Crisis 2 PARADISE LOST 3 Developing Solutions to Irans Environmental Crisis
PARADISE LOST 1 Developing solutions to Irans environmental crisis PARADISE LOST? Developing solutions to Irans environmental crisis 2 PARADISE LOST 3 Developing solutions to Irans environmental crisis PARADISE LOST? Developing solutions to Irans environmental crisis 4 About Heinrich Böll Foundation About Small Media The Heinrich Böll Foundation, affiliated with Small Media is an organisation working to the Green Party and headquartered in the support civil society development and human heart of Berlin, is a legally independent political rights advocacy in the Middle East. We do foundation working in the spirit of intellectual this by providing research, design, training, openness. The Foundation’s primary objective and technology support to partners across is to support political education both within the region, and by working with organisations Germany and abroad, thus promoting to develop effective and innovative digital democratic involvement, sociopolitical activism, advocacy strategies and campaigns. We also and cross-cultural understanding. The provide digital security support to a range of Foundation also provides support for art and partners to ensure that they can work safely culture, science and research, and and securely. development cooperation. Its activities are guided by the fundamental political values of ecology, democracy, solidarity, and non-violence. Acknowledgements Small Media and the Heinrich Böll Foundation would like to thank all of the contributors to this report, who for security purposes have chosen to remain anonymous. This research would not have been possible without their generous assistance and support. Credits Research James Marchant // Valeria Spinelli // Mo Hoseini Design Richard Kahwagi // Surasti Puri This report was printed using FSC® certified uncoated paper, made from 100% recycled pulp. -

New Towns in Iran
Young Cities – New Towns in Iran New Towns as a Concept for the Sustainable Development of Megacity Regions Final Project Report | Reporting Period: July 1st 2005 – March 31st 2008 Funding programme The Urban Transition | Research for Sustainable Development of the Megacities of Tomorrow 1 YOUNG CITIES – NEW TOWNS IN IRAN | Final Project Report | 2005 - 2008 Young Cities – New Towns in Iran | New Towns as a Concept for the Sustainable Development of Megacity Regions: Final Project Report BMBF Project funding reference number: 01LG0513 Project coordinator: Prof Dr Rudolf Schäfer | TU Berlin Final Report | Reporting Period: 01.07.2005 – 31.03.2008| Berlin, December 15th 2008 Report edited by Young Cities Project Center: Dipl.-Ing. Sebastian Seelig, Dipl.-Ing. Florian Stellmacher Joint German-Iranian Project Consortium Technische Universität Berlin Berlin, Germany Building and Housing Research Center Tehran, Islamic Republic of Iran New Towns Development Corporation Tehran, Islamic Republic of Iran Berlin-Brandenburg Construction Industry Association (BIV) e.V. Potsdam, Germany FIRST Fraunhofer Institute for Computer Architecture and Software Technology Berlin, Germany inter3 Institute for Management of Resources GmbH Berlin, Germany nexus Institute for Cooperation Management & Interdisciplinary Research GmbH Berlin, Germany p2m berlin GmbH Berlin, Germany University of the Arts Berlin Berlin, Germany Vocational Training Institute e.V. of the BIV Potsdam, Germany 3 YOUNG CITIES – NEW TOWNS IN IRAN | Final Project Report | 2005 - 2008 Table