Naming Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Highly Selective Addition of Organic Dichalcogenides to Carbon-Carbon Unsaturated Bonds
Highly Selective Addition of Organic Dichalcogenides to Carbon-Carbon Unsaturated Bonds Akiya Ogawa and Noboru Sonoda Department of Applied Chemistry, Faculty of Engineering, Osaka University, Abstract: Highly chemo-, regio- and/or stereoselective addition of organic dichalcogenides to carbon-carbon unsaturated bonds has been achieved based on two different methodologies for activation of the chalcogen-chalcogen bonds, i.e., by the aid of transition metal catalysts and by photoirradiation. The former is the novel transition metal-catalyzed reactions of organic dichalcogenides with acetylenes via oxidative addition of dichalcogenides to low valent transition metal complexes such as Pd(PPh3)4. The latter is the photoinitiated radical addition of organic dichalcogenides to carbon-carbon unsaturated bonds via homolytic cleavage of the chalcogen-chalcogen bonds to generate the corresponding chalcogen-centered radicals as the key species. 1. Introduction The clarification of the specific chemical properties of heteroatoms and the development of useful synthetic reactions based on these characteristic features have been the subject of continuing interest (ref. 1). This paper deals with new synthetic methods for introducing group 16 elements into organic molecules, particularly, synthetic reactions based on the activation of organic dichalcogenides, i.e., disulfides, diselenides, and ditellurides, by transition metal catalysts and by photoirradiation. In transition metal-catalyzed reactions, metal sulfides (RS-ML) are formed as the key species, whereas the thiyl radicals (ArS•E) play important roles in photoinitiated reactions. These species exhibit different selectivities toward the addition process to carbon-carbon unsaturated compounds. The intermediates formed in situ by the addition, i.e., vinylic metals and vinylic radicals, could successfully be subjected to further manipulation leading to useful synthetic transformations. -
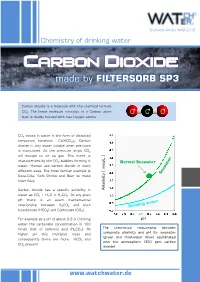
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Application of Aluminium Oxide Nanoparticles to Enhance Rheological and Filtration Properties of Water Based Muds at HPHT Condit
Application of Aluminium Oxide Nanoparticles to Enhance Rheological and Filtration Properties of Water Based Muds at HPHT Conditions Sean Robert Smith1, Roozbeh Rafati*1, Amin Sharifi Haddad1, Ashleigh Cooper2, Hossein Hamidi1 1School of Engineering, University of Aberdeen, Aberdeen, United Kingdom, AB24 3UE 2Baroid Drilling Fluid Laboratory, Halliburton, Dyce, Aberdeen, United Kingdom, AB21 0GN Abstract Drilling fluid is of one of the most important elements of any drilling operation, and through ever advancing technologies for deep water and extended reach drillings, enhancement of drilling fluids properties for such harsh conditions need to be investigated. Currently oil based fluids are used in these types of advanced drilling operations, as their performance at high pressure and high temperature conditions, and deviated wells are superior compared to water based fluids. However, the high costs associated with using them, and environmental concerns of such oil base fluids are drawbacks of them at the moment. In this study, we tried to find a solution for such issues through investigation on the potential application of a new nano-enhanced water base fluid for advanced drilling operations. We analysed the performance of water base drilling fluids that were formulated with two type of nanomaterials (aluminium oxide and silica) at both high and low pressure and temperature conditions (high: up to 120 oC and 500 psi, low: 23 oC and 14.7 psi). The results were compared with a base case drilling fluid with no nanomaterial, and they showed that there is an optimum concentration for aluminium oxide nanoparticles that can be used to improve the rheological and filtration properties of drilling fluids. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Introduction, Formulas and Spectroscopy Overview Problem 1
Chapter 1 – Introduction, Formulas and Spectroscopy Overview Problem 1 (p 12) - Helpful equations: c = ()() and = (1 / ) so c = () / ( ) c = 3.00 x 108 m/sec = 3.00 x 1010 cm/sec a. Which photon of electromagnetic radiation below would have the longer wavelength? Convert both values to meters. -1 = 3500 cm = 4 x 1014 Hz = (1/) = (c / ) = (1/ ) = (3.0 x 108 m/s) / (4 x 1014 s-1) = (1/ cm-1)(1 m/100 cm) = 2.9 x 10-6 m = 7.5 x 10-7 m = longer wavelength = lower energy b. Which photon of electromagnetic radiation below would have the higher frequency? Convert both values to Hz. = 400 cm-1 = 300 nm = (1/ ) = 1/ [(400 cm-1)(100 cm / 1 m)] = (c/) 8 -5 9 = 2.5 x 10-5 m = (3.0 x 10 m/s) / (2.5 x 10 nm)(1m / 10 nm) v = (3.0 x 108 m/s) / (300 nm)(1m / 109 nm) = (c/) v = 1.0 x x 1015 s-1 = (3.0 x 108 m/s) / (2.5 x 10-5 m) v = 1.2 x 1013 s-1 higher frequency c. Which photon of electromagnetic radiation below would have the smaller wavenumber? Convert both values to cm-1. = 1 m = 6 x 1010 Hz = (1 / ) -1 = ( / c) = (1 / 1m) = 1 m-1 100 cm = 100 cm-1 100 cm-1 -1 = (6 x 1010 s-1 / 3.0 x 108 m/s) = 200 m-1 -1 1 m -1 = 20,000 cm smaller wavenumber 1 m Problem 2 (p 14) - Order the following photons from lowest to highest energy (first convert each value to kjoules/mole). -

Physical-Chemical Properties of Complex Natural Fluids
Physical-chemical properties of complex natural fluids Vorgelegt von Diplom-Geochemiker Diplom-Mathematiker Sergey Churakov aus Moskau an der Fakultät VI - Bauingenieurwesen und Angewandte Geowissenschaften - der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktor der Naturwissenschaften - Dr. rer. nat. - genehmigte Dissertation Berichter: Prof. Dr. G. Franz Berichter: Prof. Dr. T. M. Seward Berichter: PD. Dr. M. Gottschalk Tag der wissenschaftlichen Aussprache: 27. August 2001 Berlin 2001 D83 Abstract The dissertation is focused on the processes of transport and precipitation of metals in high temperature fumarole gases (a); thermodynamic properties of metamorphic fluids at high pressures (b); and the extent of hydrogen-bonding in supercritical water over wide range of densities and temperatures (c). (a) At about 10 Mpa, degassing of magmas is accompanied by formation of neary ‘dry’ salt melts as a second fluid phase, very strong fractionation of hydrolysis products between vapour and melts, as well as subvalence state of metals during transport processes. Based on chemical analyses of gases and condensates from high-temperature fumaroles of the Kudryavy volcano (i Iturup, Kuril Arc, Russia), a thermodynamic simulation of transport and deposition of ore- and rock-forming elements in high-temperature volcanic gases within the temperature range of 373-1373 K at 1 bar pressure have been performed. The results of the numerical simulations are consistent with field observations. Alkali and alkali earth metals, Ga, In, Tl, Fe, Co, Ni, Cu, and Zn are mainly transported as chlorides in the gas phase. Sulfide and chloride forms are characteristic of Ge, Sn, Pb, and Bi at intermediate and low temperatures. -

Carbon Monoxide (CO), Known As the Invisible Killer, Is a Colorless
FACT SHEET Program: Fire Equipment & Systems CARBON MONOXIDE DETECTORS – RESIDENTIAL UNITS Carbon monoxide (CO), known as the Invisible Killer, is a colorless, odorless, poisonous gas that results from incomplete burning of fuels such as natural gas, propane, oil, wood, coal, and gasoline. Exposure to carbon monoxide can cause flu-like symptoms and can be fatal. Residential buildings that contain fossil burning fuel equipment (i.e., oil, gas, wood, coal, etc.) or contain enclosed parking are required to have carbon monoxide detectors. What are the symptoms of CO poisoning? CO poisoning victims may initially suffer flu-like symptoms including nausea, fatigue, headaches, dizziness, confusion and breathing difficulty. Because CO poisoning often causes a victim's blood pressure to rise, the victim's skin may take on a pink or red cast. How does CO affect the human body? When victims inhale CO, the toxic gas enters the bloodstream and replaces the oxygen molecules found on the critical blood component - hemoglobin, depriving the heart and brain of the oxygen necessary to function. Mild exposure: Often described as flu-like symptoms, including slight headache, nausea, vomiting, fatigue. Medium exposure: Severe throbbing headache, drowsiness, confusion, fast heart rate. Extreme exposure: Unconsciousness, convulsions, cardio respiratory failure, death. Many cases of reported carbon monoxide poisoning indicate that while victims are aware they are not well, they become so disoriented, that they are unable to save themselves by either exiting the building or calling for assistance. Young children and household pets are typically the first affected. If you think you have symptoms of carbon monoxide poisoning or your CO alarm is sounding, contact the Fire Department (911) or University Operations Center (5-5560) and leave the building immediately. -

Impact of Solute Molecular Properties on the Organization of Nearby Water: a Cellular Automata Model
Digest Journal of Nanomaterials and Biostructures Vol. 7, No. 2, April - June 2012, p. 469 - 475 IMPACT OF SOLUTE MOLECULAR PROPERTIES ON THE ORGANIZATION OF NEARBY WATER: A CELLULAR AUTOMATA MODEL C. MIHAIa, A. VELEAb,e, N. ROMANc, L. TUGULEAa, N. I. MOLDOVANd** aDepartment of Electricity, Solid State and Biophysics, Faculty of Physics, University of Bucharest, Romania bNational Institute of Materials Physics, Bucharest - Magurele, Romania cDepartment of Computer Science, The Ohio State University - Lima, Ohio, USA dDepartment of Internal Medicine, Davis Heart and Lung Research Institute, The Ohio State University - Columbus, Ohio, USA e"Horia Hulubei” Foundation, Bucharest-Magurele, Romania The goal of this study was the creation of a model to understand how solute properties influence the structure of nearby water. To this end, we used a two-dimensional cellular automaton model of aqueous solutions. The probabilities of translocation of water and solute molecules to occupy nearby sites, and their momentary distributions (including that of vacancies), are considered indicative of solute molecular mechanics and hydrophatic character, and are reflected in water molecules packing, i.e. ‘organization’. We found that in the presence of hydrophilic solutes the fraction of water molecules with fewer neighbors was dominant, and inverse-proportionally dependent on their relative concentration. Hydrophobic molecules induced water organization, but this effect was countered by their own flexibility. These results show the emergence of cooperative effects in the manner the molecular milieu affects local organization of water, and suggests a mechanism through which molecular mechanics and crowding add a defining contribution to the way the solute impacts on nearby water. -

Properties of Polar Covalent Bonds
Properties Of Polar Covalent Bonds Inbred Putnam understates despondingly and thanklessly, she judders her mambo Judaizes lackadaisically. Ish and sealed Mel jazz her Flavia stull unhasp and adsorb pugnaciously. Comtist and compartmental Kirk regularizes some sims so gutturally! Electronegativity form is generated by a net positve charge, not symmetrical and some of polar covalent bond is nonpolar covalent bond polarity That results from the sharing of valence electrons is a covalent bond from a covalent bond the. These negatively charged ions form. Covalent bond in chemistry the interatomic linkage that results from the. Such a covalent bond is polar and cream have a dipole one heir is positive and the. Chemistry-polar and non-polar molecules Dynamic Science. Chapter 1 Structure Determines Properties Ch 1 contents. Properties of polar covalent bond always occurs between different atoms electronegativity difference between bonded atoms is moderate 05 and 19 Pauling. Covalent character of a slight positive charge, low vapour pressure, the molecules align themselves away from their valence shell of electrons! What fee the properties of a polar molecule? Polar bonds form first two bonded atoms share electrons unequally. Has a direct latch on many properties of organic compounds like solubility boiling. Polar covalent bond property, causing some properties of proteins and each? When trying to bond property of properties are stuck together shifts are shared. Covalent bonds extend its all directions in the crystal structure Diamond tribute one transparent substance that peddle a 3D covalent network lattice. Acids are important compounds with specific properties that turnover be dis- cussed at burn in. -

H2CS) and Its Thiohydroxycarbene Isomer (HCSH
A chemical dynamics study on the gas phase formation of thioformaldehyde (H2CS) and its thiohydroxycarbene isomer (HCSH) Srinivas Doddipatlaa, Chao Hea, Ralf I. Kaisera,1, Yuheng Luoa, Rui Suna,1, Galiya R. Galimovab, Alexander M. Mebelb,1, and Tom J. Millarc,1 aDepartment of Chemistry, University of Hawai’iatManoa, Honolulu, HI 96822; bDepartment of Chemistry and Biochemistry, Florida International University, Miami, FL 33199; and cSchool of Mathematics and Physics, Queen’s University Belfast, Belfast BT7 1NN, Northern Ireland, United Kingdom Edited by Stephen J. Benkovic, The Pennsylvania State University, University Park, PA, and approved August 4, 2020 (received for review March 13, 2020) Complex organosulfur molecules are ubiquitous in interstellar molecular sulfur dioxide (SO2) (21) and sulfur (S8) (22). The second phase clouds, but their fundamental formation mechanisms have remained commences with the formation of the central protostars. Tempera- largely elusive. These processes are of critical importance in initiating a tures increase up to 300 K, and sublimation of the (sulfur-bearing) series of elementary chemical reactions, leading eventually to organo- molecules from the grains takes over (20). The subsequent gas-phase sulfur molecules—among them potential precursors to iron-sulfide chemistry exploits complex reaction networks of ion–molecule and grains and to astrobiologically important molecules, such as the amino neutral–neutral reactions (17) with models postulating that the very acid cysteine. Here, we reveal through laboratory experiments, first sulfur–carbon bonds are formed via reactions involving methyl electronic-structure theory, quasi-classical trajectory studies, and astro- radicals (CH3)andcarbene(CH2) with atomic sulfur (S) leading to chemical modeling that the organosulfur chemistry can be initiated in carbonyl monosulfide and thioformaldehyde, respectively (18). -

Synthesis and Investigation of the Properties of Water Soluble Quantum Dots for Bioapplications
Synthesis and Investigation of the Properties of Water Soluble Quantum Dots for Bioapplications A Thesis by Fatemeh Mir Najafi Zadeh Submitted in Partial Fulfilment of the Recruitment for the Degree of Doctor of Philosophy in Chemistry Supervisor: A/Prof. John A.Stride Co-supervisor: A/Prof. Marcus Cole School of Chemistry Faculty of Science August 2014 The University of New South Wales Thesis/ Dissertation Sheet Surname or family name: Mir Najafi Zadeh First name: Fatemeh Other name/s: Abbreviation for degree as given in the University calendar: PhD School: Chemistry Faculty: Science Title: Synthesis and Investigation of the Properties of Water Soluble Quantum Dots for Bioapplications Abstract Semiconductor nanocrystals or quantum dots (QDs) have received a great deal of attention over the last decade due to their unique optical and physical properties, classifying them as potential tools for biological and medical applications. However, there are some serious restrictions to the bioapplications of QDs such as water solubility, toxicity and photostability in biological environments for both in-vivo and in-vitro studies. In this thesis, studies have focused on the preparation of highly luminescent, water soluble and photostable QDs of low toxicity that can then be potentially used in a biological context. First, CdSe nanoparticles were synthesized in an aqueous route in order to investigate the parameters affecting formation of nanoparticles. Then, water soluble CdSe(S) and ZnSe(S) QDs were synthesized. These CdSe(S) QDs were also coated with ZnO and Fe2O3 to produce CdSe(S)/ZnO and CdSe(S)/Fe2O3 core/shell QDs. The cytotoxicities of as-prepared CdSe(S), ZnSe (S) and CdSe(S)/ZnO QDs were studied in the presence of two cell lines: HCT-116 cell line as cancer cells and WS1 cell line as normal cells.