THE ECOLOGY of SLEEP in REPTILES Authors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
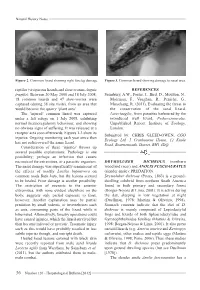
Reptiles (Viviparous Lizards and Slow-Worms Anguis REFERENCES Fragilis)
Natural History Notes Figure 2. Common lizard showing right foreleg damage. Figure 3. Common lizard showing damage to nasal area. reptiles (viviparous lizards and slow-worms Anguis REFERENCES fragilis). Between 20 May 2008 and 18 July 2008, Sainsbury, A.W., Foster, J., Bird, D., Moulton, N., 18 common lizards and 47 slow-worms were Molenaar, F., Vaughan, R., Peniche, G., captured (during 38 site visits), from an area that Marschang, R. (2011). Evaluating the threat to would become the quarry ‘plant area’. the conservation of the sand lizard, The ‘injured’ common lizard was captured Lacertaagilis, from parasites harboured by the under a felt refuge on 1 July 2008, exhibiting introduced wall lizard, Podarcismuralis. normal thermoregulatory behaviour, and showing Unpublished Report. Institute of Zoology, no obvious signs of suffering. It was released at a London. receptor area soon afterwards. Figures 1-3 show its Submitted by: CHRIS GLEED-OWEN, CGO injuries. Ongoing monitoring each year since then Ecology Ltd, 5 Cranbourne House, 12 Knole has not rediscovered the same lizard. Road, Bournemouth, Dorset, BH1 4DQ. Consideration of these ‘injuries’ throws up several possible explanations. Pathology is one possibility; perhaps an infection that causes necrosis of the extremities, or a parasitic organism. DRYMOLUBER DICHROUS (northern The nasal damage was superficially reminiscent of woodland racer) and ANOLIS FUSCOAURATUS the effects of toadfly Lucilia bufonivora on (slender anole): PREDATION. common toads Bufo bufo, but the lesions seemed Drymoluber dichrous (Peters, 1863) is a ground- to be healed. Frost damage is another possibility. dwelling colubrid from northern South America The restriction of necrosis to the anterior found in both primary and secondary forest extremities, with none evident elsewhere on the (Borges-Nojosa & Lima, 2001). -

Scale Sensillae of the File Snake (Serpentes: Acrochordidae) and Some Other Aquatic and Burrowing Snakes
SCALE SENSILLAE OF THE FILE SNAKE (SERPENTES: ACROCHORDIDAE) AND SOME OTHER AQUATIC AND BURROWING SNAKES by DAVID POVEL and JEROEN VAN DER KOOIJ (Section Dynamic Morphology,Institute of Evolutionaryand Ecological Sciences, Leiden University,P.O. Box 9516, 2300 RA Leiden, The Netherlands) ABSTRACT The acrochordid snakes are aquatic, living in environmentswith often a poor visibility. It therefore was investigatedhow these animals detect their prey. Two earlier studies of their scales revealed a rather complex scale organ, composedof hairlike protrusions and plate-like structures. However, no satisfactory explanation was given for the structures found, e.g., an undefined sensilla or a gland. Skin samples from various sites of the body of Acrochordus granulatus and A. javanicus were studied. Scanning electron microscopic pictures revealed that each scale of the head contains up to seven sensillae, and each of the keeled scales of the rest of the body has one. Also a modified Allochrome staining procedure on tissue samples was performed to detect glycogen, which is known to occur in discoidal nerve endings of tactile sense organs of reptiles. Light microscopicslides revealedglycogen particles in a small pillow-shaped area just below the hairlike protrusions of an organ. Moreover, small nerves were recognized near the same location. No indications were found for the scale organs to have a glandular function. Because of the reported reactions of a snake when it is touched by a fish, these scale sensilla are proposed to be very sensitivemechanoreceptors. Comparisons were made with the scale organs of snakes from various habitats, viz. the seasnake Lapemis hardwicki, and burrowing snakes such as Xenopeltis unicolor and Cylindrophisrufus. -

Checklist of Helminths from Lizards and Amphisbaenians (Reptilia, Squamata) of South America Ticle R A
The Journal of Venomous Animals and Toxins including Tropical Diseases ISSN 1678-9199 | 2010 | volume 16 | issue 4 | pages 543-572 Checklist of helminths from lizards and amphisbaenians (Reptilia, Squamata) of South America TICLE R A Ávila RW (1), Silva RJ (1) EVIEW R (1) Department of Parasitology, Botucatu Biosciences Institute, São Paulo State University (UNESP – Univ Estadual Paulista), Botucatu, São Paulo State, Brazil. Abstract: A comprehensive and up to date summary of the literature on the helminth parasites of lizards and amphisbaenians from South America is herein presented. One-hundred eighteen lizard species from twelve countries were reported in the literature harboring a total of 155 helminth species, being none acanthocephalans, 15 cestodes, 20 trematodes and 111 nematodes. Of these, one record was from Chile and French Guiana, three from Colombia, three from Uruguay, eight from Bolivia, nine from Surinam, 13 from Paraguay, 12 from Venezuela, 27 from Ecuador, 17 from Argentina, 39 from Peru and 103 from Brazil. The present list provides host, geographical distribution (with the respective biome, when possible), site of infection and references from the parasites. A systematic parasite-host list is also provided. Key words: Cestoda, Nematoda, Trematoda, Squamata, neotropical. INTRODUCTION The present checklist summarizes the diversity of helminths from lizards and amphisbaenians Parasitological studies on helminths that of South America, providing a host-parasite list infect squamates (particularly lizards) in South with localities and biomes. America had recent increased in the past few years, with many new records of hosts and/or STUDIED REGIONS localities and description of several new species (1-3). -

Snakes of the Siwalik Group (Miocene of Pakistan): Systematics and Relationship to Environmental Change
Palaeontologia Electronica http://palaeo-electronica.org SNAKES OF THE SIWALIK GROUP (MIOCENE OF PAKISTAN): SYSTEMATICS AND RELATIONSHIP TO ENVIRONMENTAL CHANGE Jason J. Head ABSTRACT The lower and middle Siwalik Group of the Potwar Plateau, Pakistan (Miocene, approximately 18 to 3.5 Ma) is a continuous fluvial sequence that preserves a dense fossil record of snakes. The record consists of approximately 1,500 vertebrae derived from surface-collection and screen-washing of bulk matrix. This record represents 12 identifiable taxa and morphotypes, including Python sp., Acrochordus dehmi, Ganso- phis potwarensis gen. et sp. nov., Bungarus sp., Chotaophis padhriensis, gen. et sp. nov., and Sivaophis downsi gen. et sp. nov. The record is dominated by Acrochordus dehmi, a fully-aquatic taxon, but diversity increases among terrestrial and semi-aquatic taxa beginning at approximately 10 Ma, roughly coeval with proxy data indicating the inception of the Asian monsoons and increasing seasonality on the Potwar Plateau. Taxonomic differences between the Siwalik Group and coeval European faunas indi- cate that South Asia was a distinct biogeographic theater from Europe by the middle Miocene. Differences between the Siwalik Group and extant snake faunas indicate sig- nificant environmental changes on the Plateau after the last fossil snake occurrences in the Siwalik section. Jason J. Head. Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, Washington, DC 20013-7012, USA. [email protected] School of Biological Sciences, Queen Mary, University of London, London, E1 4NS, United Kingdom. KEY WORDS: Snakes, faunal change, Siwalik Group, Miocene, Acrochordus. PE Article Number: 8.1.18A Copyright: Society of Vertebrate Paleontology May 2005 Submission: 3 August 2004. -

<I>ANOLIS</I> LIZARDS in the FOOD WEBS of STRUCTURALLY
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2016 ASSESSING THE FUNCTIONAL SIMILARITY OF NATIVE AND INVASIVE ANOLIS LIZARDS IN THE FOOD WEBS OF STRUCTURALLY-SIMPLE HABITATS IN FLORIDA Nathan W. Turnbough University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Terrestrial and Aquatic Ecology Commons Recommended Citation Turnbough, Nathan W., "ASSESSING THE FUNCTIONAL SIMILARITY OF NATIVE AND INVASIVE ANOLIS LIZARDS IN THE FOOD WEBS OF STRUCTURALLY-SIMPLE HABITATS IN FLORIDA. " PhD diss., University of Tennessee, 2016. https://trace.tennessee.edu/utk_graddiss/4174 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Nathan W. Turnbough entitled "ASSESSING THE FUNCTIONAL SIMILARITY OF NATIVE AND INVASIVE ANOLIS LIZARDS IN THE FOOD WEBS OF STRUCTURALLY-SIMPLE HABITATS IN FLORIDA." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Ecology and Evolutionary Biology. -

Sleep-Site Fidelity in Cuban Green
HTTPS://JOURNALS.KU.EDU/REPTILESANDAMPHIBIANSTABLE OF CONTENTS IRCF REPTILES & AMPHIBIANSREPTILES • VOL & AMPHIBIANS15, NO 4 • DEC 2008 • 28(2):189 245–247 • AUG 2021 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS Sleep-siteFEATURE ARTICLES Fidelity in Cuban Green Anoles, . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: On the Road Anolisto Understanding the Ecology porcatus and Conservation of the Midwest’s Gray Giant Serpent ......................1840 Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: A Hypothetical Excursion(Squamata: ............................................................................................................................ Dactyloidae)Robert W. Henderson 198 RESEARCH ARTICLES . The Texas Horned Lizard in Central and Western TexasLuis ....................... F. de Armas Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida .............................................P.O. Box 4327, SanBrian Antonio J. Camposano, de los Baños,Kenneth ArtemisaL. Krysko, Kevin38100, M. CubaEnge, Ellen([email protected]) M. Donlan, and Michael Granatosky 212 CONSERVATION ALERT Photographs by the author. World’s Mammals in Crisis ............................................................................................................................................................. 220 . More Than Mammals ..................................................................................................................................................................... -

An Annotated Type Catalogue of the Dragon Lizards (Reptilia: Squamata: Agamidae) in the Collection of the Western Australian Museum Ryan J
RECORDS OF THE WESTERN AUSTRALIAN MUSEUM 34 115–132 (2019) DOI: 10.18195/issn.0312-3162.34(2).2019.115-132 An annotated type catalogue of the dragon lizards (Reptilia: Squamata: Agamidae) in the collection of the Western Australian Museum Ryan J. Ellis Department of Terrestrial Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia. Biologic Environmental Survey, 24–26 Wickham St, East Perth, Western Australia 6004, Australia. Email: [email protected] ABSTRACT – The Western Australian Museum holds a vast collection of specimens representing a large portion of the 106 currently recognised taxa of dragon lizards (family Agamidae) known to occur across Australia. While the museum’s collection is dominated by Western Australian species, it also contains a selection of specimens from localities in other Australian states and a small selection from outside of Australia. Currently the museum’s collection contains 18,914 agamid specimens representing 89 of the 106 currently recognised taxa from across Australia and 27 from outside of Australia. This includes 824 type specimens representing 45 currently recognised taxa and three synonymised taxa, comprising 43 holotypes, three syntypes and 779 paratypes. Of the paratypes, a total of 43 specimens have been gifted to other collections, disposed or could not be located and are considered lost. An annotated catalogue is provided for all agamid type material currently and previously maintained in the herpetological collection of the Western Australian Museum. KEYWORDS: type specimens, holotype, syntype, paratype, dragon lizard, nomenclature. INTRODUCTION Australia was named by John Edward Gray in 1825, The Agamidae, commonly referred to as dragon Clamydosaurus kingii Gray, 1825 [now Chlamydosaurus lizards, comprises over 480 taxa worldwide, occurring kingii (Gray, 1825)]. -

1919 Barbour the Herpetology of Cuba.Pdf
5 2^usniuc^££itt£j IK: Bofji^D ^Jae HARVARD UNIVERSITY. I LIBRARY OF THE MUSEUM OF COMPARATIVE ZOOLOGY GIFT OF jflDemoirs of tbe /IDuseum of Comparattve ZooloQ? AT HARVARD COLLEGE. Vol. XLVII. No. 2. THE HRKFETOLOGY OF CUBA. BY THOMAS BARBOUR AND CHARLES T. RAMSDEN. WITH FIFTEEN PLATES. CAMBRIDGE, U. S. A.: IPrlnteb for tbe jIDuseum, May, 1919. i /IDemotta of tbe /iDuseum of Comparattve Zoology AT HARVARD COLLEGE. Vol. XLVII. No. 2. (./,!', THE HERPETOLOGY OF CUBA. BY THOMAS BARBOUR AND CHARLES T. RAMSDEN. WITH FIFTEEN PLATES. CAMBRIDGE, U. S. A.: Iprtnteft for tbe /IDuseum. May, 1919. {^A CONTENTS. Page Page Introduction 73 Gonatodes fuscus (Hallowell), Synopsis of the species 73 Plate 1, fig. 5 114 Species erroneously recorded .... 75 Tarentola cubana (Gundlach & Geographic note 78 Peters), Plate 14, fig. 1 . 116 Faiinal relationships 79 Hemidactylus mabouia (Moreau Check list of the species 83 de Jonnes) 117 Systematic account of the species . 93 Key to the species of Sphaerodactylus 1 19 Keys 93 Sphaerodactylus torrei Barbour, Amphibia Salientia 93 Plate 2, fig. 1, 2 119 Key to the genera 93 elegans MacLea>', Plate 2, fig. 3 121 Hylidae 93 cinereus MacLeay, Plate 2, fig. 4 122 Hyla septentrionalis Boulenger, nigropunctatus Gray, Plate 3, 1 Plate 1, fig. 1 93 fig. 124 Bufonidae 95 notatus Baird, Plate 3, fig. 2 125 Key to the species of Bufo ... 95 *scaber Barbour & Ramsden Plate Bufo longinasus Stejneger, Plate 3, fig. 3 126 13, fig. 1 ....... 95 Iguanidae 128 ramsdeni Barbour, Plate 1, fig. 2 96 Chamaeleolis chamaeleontides peltacephalus Tschudi, Plate 13, (Dmneril & Bibron) Plate 14, fig. -

Stellio' in Herpetology and a Comment on the Nomenclature and Taxonomy of Agamids of the Genus Agama (Sensu Lato) (Squamata: Sauria: Agamidae)
©Österreichische Gesellschaft für Herpetologie e.V., Wien, Austria, download unter www.biologiezentrum.at HERPETOZOA 8 (1/2): 3 - 9 Wien, 30. Juli 1995 A brief review of the origin and use of 'stellio' in herpetology and a comment on the nomenclature and taxonomy of agamids of the genus Agama (sensu lato) (Squamata: Sauria: Agamidae) Kurzübersicht über die Herkunft und Verwendung von "stellio" in der Herpetologie und Kommentar zur Nomenklatur und Taxonomie von Agamen, Gattung Agama (sensu lato) (Squamata: Sauria: Agamidae) KLAUS HENLE ABSTRACT The name 'stellio' has received a wide and variable application in herpetology. Its use antedates modern nomenclature. The name was applied mainly to various agamid and gekkonid species. In modern usage, confu- sion exists primarily with its use as a genus, i. e., Siellio. To solve this problem, STEJNEGER (1936) desig- nated Siellio saxatilis of LAURENTI, 1768 which is based on a figure in SEBA (1734) as the type species. This species is unidentifiable. In an unpublished thesis, MOODY (1980) split the genus Agama (s. 1.) into six genera. He overlooked STEJNEGER's (1936) designation and reused Stellio for the stellio-group of agamid lizards. Many authors followed MOODY (1980). Recently, some authors pointed out that Siellio is unavailable but did not fully dis- cuss the implications for agamid nomenclature. It is argued that a satisfactory nomenclature is difficult with current knowledge of agamid taxonomy. It is suggested to restrict Laudakia to L. tuberculata and to use Ploce- denna for the stellio-group (sensu stricto). KURZFASSUNG Der Name "stellio" sah eine breite und vielfältige Verwendung in der Herpetologie, die weit über die moderne Nomenklatur zurückreicht. -

The Herpetofauna of the Cubango, Cuito, and Lower Cuando River Catchments of South-Eastern Angola
Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 10(2) [Special Section]: 6–36 (e126). The herpetofauna of the Cubango, Cuito, and lower Cuando river catchments of south-eastern Angola 1,2,*Werner Conradie, 2Roger Bills, and 1,3William R. Branch 1Port Elizabeth Museum (Bayworld), P.O. Box 13147, Humewood 6013, SOUTH AFRICA 2South African Institute for Aquatic Bio- diversity, P/Bag 1015, Grahamstown 6140, SOUTH AFRICA 3Research Associate, Department of Zoology, P O Box 77000, Nelson Mandela Metropolitan University, Port Elizabeth 6031, SOUTH AFRICA Abstract.—Angola’s herpetofauna has been neglected for many years, but recent surveys have revealed unknown diversity and a consequent increase in the number of species recorded for the country. Most historical Angola surveys focused on the north-eastern and south-western parts of the country, with the south-east, now comprising the Kuando-Kubango Province, neglected. To address this gap a series of rapid biodiversity surveys of the upper Cubango-Okavango basin were conducted from 2012‒2015. This report presents the results of these surveys, together with a herpetological checklist of current and historical records for the Angolan drainage of the Cubango, Cuito, and Cuando Rivers. In summary 111 species are known from the region, comprising 38 snakes, 32 lizards, five chelonians, a single crocodile and 34 amphibians. The Cubango is the most western catchment and has the greatest herpetofaunal diversity (54 species). This is a reflection of both its easier access, and thus greatest number of historical records, and also the greater habitat and topographical diversity associated with the rocky headwaters. -

A Further Break-Up of the Australian Gecko Genus
Australasian Journal of Herpetology 3 Australasian Journal of Herpetology 34:3-35. ISSN 1836-5698 (Print) Published 20 July 2017. ISSN 1836-5779 (Online) A further break-up of the Australian gecko genus Oedura Gray, 1842 sensu lato as currently recognized, from four to seven genera, with two new subgenera defined, description of fourteen new species, four new subspecies and formalising of one tribe and five subtribes. RAYMOND T. HOSER 488 Park Road, Park Orchards, Victoria, 3134, Australia. Phone: +61 3 9812 3322 Fax: 9812 3355 E-mail: snakeman (at) snakeman.com.au Received 15 January 2017, Accepted 20 May 2017, Published 20 July 2017. ABSTRACT The genus Oedura Gray, 1842 sensu lato has been the subject of numerous taxonomic reviews in recent years. These have resulted in division of the genus into deeply divergent, but distantly related groups at the genus level as well as numerous new species being formally named. In light of the preceding and including results of molecular studies indicating significant divergence between species groups within Oedura as recognized in 2012 and 2016, the genus as recognized prior to 2012 is further divided to become seven (from four in 2016). These all have known divergences well in excess of 15 MYA, making genus-level subdivision inevitable. Divergent subgenera with divergences in the order of 13-15 MYA are also formally named for the first time. Within this new generic arrangement, fourteen new species are formally described for the first time in accordance with the International Code of Zoological Nomenclature (Ride et al. 1999) on the basis of obvious morphological differences from similar species, which they have been treated as until now and also based on the known genetic divergences ascertained from earlier cited literature, all of which are measured in the millions of years (2.5 MYA or more). -

Analysis of Diet Composition and Morphological Characters of The
Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [General Section]: 111–121 (e172). Analysis of diet composition and morphological characters of the little-known Peruvian bush anole Polychrus peruvianus (Noble, 1924) in a northern Peruvian dry forest 1Antonia Beuttner and 2,*Claudia Koch 1Universität Tübingen, Geschwister-Scholl-Platz, 72074 Tübingen, GERMANY 2Zoologisches Forschungsmuseum Alexander Koenig (ZFMK), Adenauerallee 160, 53113 Bonn, GERMANY Abstract.—Analyses of diet composition are an important element of studies focusing on biological diversity, ecology, and behavior of animals. Polychrus peruvianus was found to be a quite abundant lizard species in the northern Peruvian dry forest. However, our knowledge of the ecology of this species remains limited. Herein, we analyze the species dietary composition and the morphological features that may be related to the feeding behavior. Our results show that P. peruvianus is a semi-herbivorous food generalist, which also consumes faunistic prey. All age groups prefer mobile prey as sit-and-wait predators. However, during ontogenesis, plant material becomes the main component in the diet of adult specimens. Keywords. Iguania, bite force, ontogenetic change, foraging strategy, stomach contents, food niche, feeding behavior, Marañón river Citation: Beuttner A, Koch C. 2019. Analysis of diet composition and morphological characters of the little-known Peruvian bush anole Polychrus peruvianus (Noble, 1924) in a northern Peruvian dry forest. Amphibian & Reptile Conservation 13(1) [General Section]: 111–121 (e172). Copyright: © 2019 Beuttner and Koch. This is an open access article distributed under the terms of the Creative Commons Attribution License [At- tribution 4.0 International (CC BY 4.0): https://creativecommons.org/licenses/by/4.0/], which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.