2010 ANNUAL REPORT Global Developer and Manufacturer of Minimally Invasive Devices for Hemorrhagic and Ischemic Stroke
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should Be Applied to the Sellers of Pharmaceutical Products? Richard C
Kentucky Law Journal Volume 78 | Issue 4 Article 3 1990 Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products? Richard C. Ausness University of Kentucky Follow this and additional works at: https://uknowledge.uky.edu/klj Part of the Consumer Protection Law Commons, and the Food and Drug Law Commons Right click to open a feedback form in a new tab to let us know how this document benefits you. Recommended Citation Ausness, Richard C. (1990) "Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products?," Kentucky Law Journal: Vol. 78 : Iss. 4 , Article 3. Available at: https://uknowledge.uky.edu/klj/vol78/iss4/3 This Article is brought to you for free and open access by the Law Journals at UKnowledge. It has been accepted for inclusion in Kentucky Law Journal by an authorized editor of UKnowledge. For more information, please contact [email protected]. Kentucky Law Journal Volume 78 1989-90 Number 4 Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products? BY RICHARD C. AusNEss* Table of Contents INTRODUCTION ....................................... 706 I. OVERVIEW ................................ 709 A. Strict Products Liability .............................. 709 B. Comment K and "Unavoidably Unsafe" Products ................................................... 712 II. COM[ENT K's LIABILITY REGm .......................... 719 A. Risks Associated with the Production Process. 720 B. Risks Arising from the Inherent Nature of a Product .................................................... 723 C. Risks Created by Conscious Design Choices .... 726 D. Scientifically Unknowable Risks ................... 731 E. -

05/01/02 Louisiana Medicaid Management
APPENDIX C 05/01/02 LOUISIANA MEDICAID MANAGEMENT INFORMATION SYSTEM PAGE 1 DEPT OF HEALTH AND HOSPITALS - BUREAU OF HEALTH SERVICES FINANCING LOUISIANA MEDICAID PHARMACY BENEFITS MANAGEMENT UNIT ONLY THESE COMPANIES PRODUCTS ARE COVERED AND ONLY THOSE DOSAGE FORMS LISTED IN APPENDIX A. MEDICAID DRUG FEDERAL REBATE PARTICIPATING PHARMACEUTICAL COMPANIES LABELER PHARMACEUTICAL COMPANY EFFECTIVE END DATE CODE DATE 00002 ELI LILLY & CO 04/01/91 00003 E.R.SQUIBB & SONS,INC 04/01/91 00004 HOFFMAN-LA ROCHE,INC 04/01/91 00005 LEDERLE LABORATORIES AMERICAN CYANAMID 04/01/91 00006 MERCK SHARP & DOHME 04/01/91 00007 SMITHKLINE BEECHAM CORPORATION 04/01/91 00008 WYETH LABORATORIES 04/01/91 00009 THE UPJOHN COMPANY 04/01/91 00011 BECTON DICKINSON MICROBIOLOGY SYSTEMS 10/01/91 07/01/98 00013 ADRIA LABORATORIES DIV.OF ERBAMONT,INC 04/01/91 00014 G.D.SEARLE & CO 04/01/91 01/01/01 00015 MEAD JOHNSON & COMPANY 04/01/91 00016 KABI PHARMACIA 04/01/91 00021 REED & CARNRICK 10/01/96 01/01/97 00023 ALLERGAN,INC 04/01/91 00024 WINTHROP PHARMACEUTICALS 04/01/91 00025 G.D.SEARLE & CO 04/01/91 00026 MILES INC.,PHARMACEUTICAL DIVISION 04/01/91 00028 GEIGY PHARMACEUTICALS 04/01/91 00029 SMITHKLINE BEECHAM CORPORATION 04/01/91 00031 ROBINS,A.H. 04/01/91 00032 SOLVAY PHARMACEUTICALS 04/01/91 00033 SYNTEX 04/01/91 00034 THE PURDUE FREDERICK COMPANY 04/01/91 00037 CARTER-WALLACE,INC 04/01/91 00038 STUART PHARMACEUTICALS,ICI AMERICAS INC 04/01/91 07/01/01 00039 HOECHST-ROUSSEL PHARMACEUTICALS INC 04/01/91 00043 SANDOZ CONSUMER CORPORATION 04/01/91 00044 KNOLL PHARMACEUTICALS -

Gender, the Status of Women, and Family Structure in Malaysia
Malaysian Journal of EconomicGender, Studies the Status 53(1): of Women,33 - 50, 2016 and Family Structure in Malaysia ISSN 1511-4554 Gender, the Status of Women, and Family Structure in Malaysia Charles Hirschman* University of Washington, Seattle Abstract: This paper addresses the question of whether the relatively high status of women in pre-colonial South-east Asia is still evident among Malay women in twentieth century Peninsular Malaysia. Compared to patterns in East and South Asia, Malay family structure does not follow the typical patriarchal patterns of patrilineal descent, patrilocal residence of newly married couples, and preference for male children. Empirical research, including ethnographic studies of gender roles in rural villages and demographic surveys, shows that women were often economically active in agricultural production and trade, and that men occasionally participated in domestic roles. These findings do not mean a complete absence of patriarchy, but there is evidence of continuity of some aspects of the historical pattern of relative gender equality. The future of gender equality in Malaysia may depend as much on understanding its past as well as drawing lessons from abroad. Keywords: Family, gender, marriage, patriarchy, women JEL classification: I3, J12, J16, N35 1. Introduction In the introduction to her book onWomen, Politics, and Change, Lenore Manderson (1980) said that the inspiration for her study was the comment by a British journalist that the participation of Malay women in rallies, demonstrations, and the nationalist movement during the late 1940s was the most remarkable feature of post-World War II Malayan politics. The British journalist described the role of Malay women in the nationalist movement as “challenging, dominant, and vehement in their emergence from meek, quiet roles in the kampongs, rice fields, the kitchens, and nurseries” (Miller, 1982, p. -

Health Industry Business Communications Council
Health Industry Business Communications Council Registered Labelers: Accredited Auto-ID Labeling Standards Argentina New MedTek Devices Pty Ltd Oxavita SRL Norseld Pty Ltd. Novadien Healthcare Pty Ltd The following companies Odontit S.A. (and/or their subsidiaries/ PAMPAMED S.R.L. Numedico Technologies Pty Ltd divisions) have applied PATEJIM SRL Opto Global Pty. Ltd. for a Labeler Identification Orthocell Limited Code (LIC) assignment with Austria Prolotus Technologies Pty Ltd HIBCC*. By doing so, they afreeze GmbH Red Milawa Pty Ltd dba Magic Mobility have demonstrated their AMI GmbH SDI Limited commitment to patient safety Bender Medsystems GmbH Signostics Ltd. and logistical efficiency for BHS Technologies GmbH Sirtex Medical Pty Ltd their customers, the industry Metasys Medizintechnik GmbH Smith & Nephew Surgical Pty. Ltd. and the public at large. PAA Laboratories GmbH Staminalift International Limited Safersonic Medizinprodukte Handels The Pipette Company Pty. Ltd. Any organization that is GmbH Thermo Electron Corporation interested in using the HIBC W & H Dentalwerk Burmoos GmbH Vush Pty Ltd uniform labeling system may apply for the assignment of VUSH STIMULATION Australia one or more LICs. William A Cook Australia Pty. Ltd. Adv. Surgical Design & Manufacture, Ltd. Last updated 9-21-2021 AirPhysio Pty Ltd Belgium Annalise-AI Pty Ltd 3M Europe Apollo Medical Imaging Technology Pty Advanced Medical Diagnostics SA/NV Ltd Analis SA/NV Benra Pty Ltd dba Gelflex Laboratories Baxter World Trade Bioclone Australia Pty. Ltd. Bio-Rad RSL Candelis, Inc. Bio-Rad Lab Inc Clinical Diag. Group DePuy Australia Pty. Ltd. Biosource Europe SA For more information, please dorsaVi Ltd Cilag NV contact the HIBCC office at: EC Certification Service GmbH Coris Bioconcept Fink Engineering Pty Ltd Fuji Hunt Photographic Chemicals NV 2525 E. -

Popular Sweeteners and Their Health Effects Based Upon Valid Scientific Data
Popular Sweeteners and Their Health Effects Interactive Qualifying Project Report Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for the Degree of Bachelor of Science By __________________________________ Ivan Lebedev __________________________________ Jayyoung Park __________________________________ Ross Yaylaian Date: Approved: __________________________________ Professor Satya Shivkumar Abstract Perceived health risks of artificial sweeteners are a controversial topic often supported solely by anecdotal evidence and distorted media hype. The aim of this study was to examine popular sweeteners and their health effects based upon valid scientific data. Information was gathered through a sweetener taste panel, interviews with doctors, and an on-line survey. The survey revealed the public’s lack of appreciation for sweeteners. It was observed that artificial sweeteners can serve as a low-risk alternative to natural sweeteners. I Table of Contents Abstract .............................................................................................................................................. I Table of Contents ............................................................................................................................... II List of Figures ................................................................................................................................... IV List of Tables ................................................................................................................................... -

Vers Un Modèle D'entreprise Hybride: L'évolution De La Gestion D'un Contexte De Loyauté Réciproque Vers Un Modèle De Marchés; Étude Du Cas De Johnson & Johnson
UNIVERSITÉ DU QUÉBEC À MONTRÉAL VERS UN MODÈLE D'ENTREPRISE HYBRIDE: L'ÉVOLUTION DE LA GESTION D'UN CONTEXTE DE LOYAUTÉ RÉCIPROQUE VERS UN MODÈLE DE MARCHÉS; ÉTUDE DU CAS DE JOHNSON & JOHNSON MÉMOIRE PRÉSENTÉ COMME EXIGENCE PARTIELLE DELA MAÎTRISE EN ADMINISTRATION DES AFFAIRES (MBA-RECHERCHE) PAR FRÉDÉRIC TREMBLAY Novembre 2008 UNIVERSITÉ DU QUÉBEC À MONTRÉAL Service des bibliothèques Avertissement La diffusion de ce mémoire se fait dans le respect des droits de son auteur, qui a signé le formulaire Autorisation de reproduire et de diffuser un travail de recherche de cycles supérieurs (SDU-522 - Rév.01-2006). Cette autorisation stipule que «conformément à l'article 11 du Règlement no 8 des études de cycles supérieurs, [l'auteur] concède à l'Université du Québec à Montréal une licence non exclusive d'utilisation et de publication de la totalité ou d'une partie importante de [son] travail de recherche pour des fins pédagogiques et non commerciales. Plus précisément, [l'auteur] autorise l'Université du Québec à Montréal à reproduire, diffuser, prêter, distribuer ou vendre des copies de [son] travail de recherche à des fins non commerciales sur quelque support que ce soit, y compris l'Internet. Cette licence et cette autorisation n'entraînent pas une renonciation de [la] part [de l'auteur] à [ses] droits moraux ni à [ses] droits de propriété intellectuelle. Sauf entente contraire, [l'auteur] conserve la liberté de diffuser et de commercialiser ou non ce travail dont [il] possède un exemplaire.» REMERCIEMENTS La réalisation d'un mémoire de maîtrise est un travail exigeant, mais très formateur. Il pousse au dépassement de soi et à la réalisation de rêves autrefois formulés. -

Sweeteners Georgia Jones, Extension Food Specialist
® ® KFSBOPFQVLCB?O>PH>¨ FK@LIKUQBKPFLK KPQFQRQBLCDOF@RIQROB>KA>QRO>IBPLRO@BP KLTELT KLTKLT G1458 (Revised May 2010) Sweeteners Georgia Jones, Extension Food Specialist Consumers have a choice of sweeteners, and this NebGuide helps them make the right choice. Sweeteners of one kind or another have been found in human diets since prehistoric times and are types of carbohy- drates. The role they play in the diet is constantly debated. Consumers satisfy their “sweet tooth” with a variety of sweeteners and use them in foods for several reasons other than sweetness. For example, sugar is used as a preservative in jams and jellies, it provides body and texture in ice cream and baked goods, and it aids in fermentation in breads and pickles. Sweeteners can be nutritive or non-nutritive. Nutritive sweeteners are those that provide calories or energy — about Sweeteners can be used not only in beverages like coffee, but in baking and as an ingredient in dry foods. four calories per gram or about 17 calories per tablespoon — even though they lack other nutrients essential for growth and health maintenance. Nutritive sweeteners include sucrose, high repair body tissue. When a diet lacks carbohydrates, protein fructose corn syrup, corn syrup, honey, fructose, molasses, and is used for energy. sugar alcohols such as sorbitol and xytilo. Non-nutritive sweet- Carbohydrates are found in almost all plant foods and one eners do not provide calories and are sometimes referred to as animal source — milk. The simpler forms of carbohydrates artificial sweeteners, and non-nutritive in this publication. are called sugars, and the more complex forms are either In fact, sweeteners may have a variety of terms — sugar- starches or dietary fibers.Table I illustrates the classification free, sugar alcohols, sucrose, corn sweeteners, etc. -

137814.Pdf (857.3Kb)
UNIVERSIDAD PANAMERICANA FACULTAD DE FILOSOFÍA Y CIENCIAS SOCIALES ESCUELA DE PEDAGOGÍA HR Redesing C A S O Q U E P R E S E N T A ALEXIS TANIA CORELLA RAMÍREZ P A R A O B T E N E R E L G R A D O D E : MAESTRA EN CAPITAL HUMANO DIRECTOR DE LA TESIS: Dr. DAVID RENE THIERRY GARCÍA MÉXICO, D.F. 17 DE DICIEMBRE DEL 2012 Universidad Panamericana 2 HR Redesing Índice Introducción ....................................................................................................................................................... 3 Marco Contextual ............................................................................................................................................... 4 Historia de Johnson & Johnson ................................................................................................................... 4 Medical Devices & Diagnostics .................................................................................................................... 5 Global Surgery Group ................................................................................................................................. 5 Global Medical Solutions ............................................................................................................................. 6 Global Orthopaedics Group ......................................................................................................................... 6 Filosofía Institucional – Nuestro Credo ..................................................................................................... -
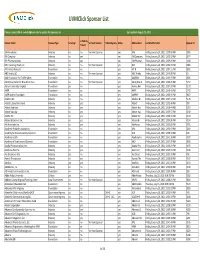
Sponsor List
UVMClick Sponsor List Please contact SPA at [email protected] to update the Sponsor List last updated August 24, 2021 Is Publicly Sponsor Name Sponsor Type Is Foreign Vermont Sponsor Federal Agency Active Abbreviation Last Modified Date Sponsor ID Traded 106 Associates Industry no no Vermont Sponsor yes 106 Friday, January 29, 2021 12:35:03 PM 3385 3M Company Industry no yes yes 3M Company Friday, January 29, 2021 12:32:32 PM 2877 3M Pharmaceuticals Industry no yes yes 3M Pharmac Friday, January 29, 2021 12:39:23 PM 4188 835 Hinesburg Road, LLC Industry no no Vermont Sponsor yes 835 Friday, January 29, 2021 12:32:33 PM 3386 A Territory Resource Foundation no no yes A.T.R. Friday, January 29, 2021 12:34:11 PM 3805 A&C Realty LLC. Industry no no Vermont Sponsor yes A&C Realty Friday, January 29, 2021 12:40:28 PM 60 AAA Foundation for Traffic Safety Foundation no no yes AAAFDN Friday, January 29, 2021 12:36:49 PM 3806 AALV/New Farms for New Americans Foundation no no Vermont Sponsor yes AALV/New FFriday, January 29, 2021 12:32:25 PM 5154 Aarhus University Hospital Foundation yes no yes Aarhus Uni Friday, January 29, 2021 12:34:23 PM 5172 AARP Foundation no no yes AARP Friday, January 29, 2021 12:40:40 PM 2742 AARP Andrus Foundation Foundation no no yes AARPAF Friday, January 29, 2021 12:36:35 PM 3807 Abalone Bio Industry no no yes Abalone Bi Friday, January 29, 2021 12:37:14 PM 5178 Abbott Laboratories Fund Industry no yes yes Abbott Friday, January 29, 2021 12:32:46 PM 309 Abbott Nutrition Industry no yes yes Abbott Nut Friday, January 29, 2021 12:38:44 PM 5219 Abbott Vascular Industry no yes yes Abbott Vas Friday, January 29, 2021 12:35:57 PM 4192 AbbVie Inc. -

Starbucks Vs. Equal Exchange: Assessing the Human Costs of Economic Globalization
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nebraska Anthropologist Anthropology, Department of 1997 Starbucks vs. Equal Exchange: Assessing the Human Costs of Economic Globalization Lindsey M. Smith Follow this and additional works at: https://digitalcommons.unl.edu/nebanthro Part of the Anthropology Commons Smith, Lindsey M., "Starbucks vs. Equal Exchange: Assessing the Human Costs of Economic Globalization" (1997). Nebraska Anthropologist. 111. https://digitalcommons.unl.edu/nebanthro/111 This Article is brought to you for free and open access by the Anthropology, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Nebraska Anthropologist by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Starbucks vs. Equal Exchange: Assessing the Human Costs of Economic Globalization Lindsey M. Smith This paper discusses the impact of economic globalization on human populations and their natural environment. Trends leading to globalization, such as multilateral and bilateral trade 8fT88ments which reduce trading barriers between countries, are discussed. According to the economic principle of comparative advantage, all countries which specialize in what they can produce most efficiently should benefit equally from fair trade. Developing countries must increasingly rely on cheap labor and low environmental standards to compete for foreign investment and capital in the global economy. Observers argue that the market is not free enough to conect the long-term damage associated with export policies like this. Poverty, misery and social stratification are increasing in many developing countries as a result. A case study of the coffee industry in Latin America provides evidence of the consequences of globalization policies on the most vulnerable populations. -

Eric M. White, O.D., Inc
ERIC M. WHITE, O.D., INC . 5075 Ruffin Road Suite B, San Diego, CA 92123 (858) 278-4720 Toll Free (866) 458-2062 FAX (858) 278-3640 [email protected] www.drericwhite.com California License #8611T STATEMENT OF CURRICULUM VITAE EDUCATION June 1982 Bachelor of Arts, Physiological Psychology University of California, San Diego; San Diego, California. May 1984 Bachelor of Science, Visual Science Southern California College of Optometry; Fullerton, California. May 1986 Doctor of Optometry Southern California College of Optometry; Fullerton, California. PRIVATE PRACTICE 1986 - 1989 Partnership; D.M. Rasmussen, O.D., F.A.A.O., Mission Village Medical Center, 3303 Ruffin Road, San Diego, California. 1989-2001 Private Practice Mission Village Medical Center, 3303 Ruffin Road, San Diego, California. 2001-Present Private Practice 5075 Ruffin Road, Suite B, San Diego, California CLINICAL ROTATIONS 1985 Southern California College of Optometry; Fullerton, California . Provided comprehensive vision care including contact lenses, vision therapy, low vision, ocular photography, electrodiagnostic services, auto perimetry, and disease detection. 1985 San Bernardino Juvenile Hall; San Bernardino, California. Provided screening and treatment of vision and learning disabilities among juveniles. 1985 D.M. Rasmussen, O.D., F.A.A.O.; San Diego, California. Assisted doctor on investigational studies, comprehensive vision care, contact lenses, low vision, autoperimetry, and disease detection . INTERNSHIP 1986 United States Naval Submarine Base, San Diego, California. Provided comprehensive vision care including fitting and care for extended wear contact lenses. 1986 U.S. Naval Medical Corps. Marine Corps Recruit Depot; San Diego, California. Provided comprehensive vision care including disease detection. 1986 Balboa Regional Naval Hospital San Diego, California. -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges.