On Evolution and Taxonomy Within the Family
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ANTIOXIDANT CAPACITY in the HEMOLYMPH of the MARINE ISOPOD PENTIDOTEA RESECATA by Leah E. Dann a THESIS Submitted to WALLA WALL
ANTIOXIDANT CAPACITY IN THE HEMOLYMPH OF THE MARINE ISOPOD PENTIDOTEA RESECATA By Leah E. Dann A THESIS submitted to WALLA WALLA UNIVERSITY in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE April 26, 2017 ABSTRACT The isopod Pentidotea resecata inhabits Zostera marina eelgrass beds. Examination of oxygen levels in a Z. marina bed indicated that P. resecata frequently experience hyperoxia and potential hypoxia reperfusion events in these beds, which may lead to enhanced reactive oxygen species (ROS) production and increased oxidative damage if the antioxidant defenses cannot sufficiently suppress these toxic oxygen intermediates. The total antioxidant capacity of P. resecata hemolymph was compared to that of Ligia pallasii, a semi-terrestrial isopod living in normoxic conditions, and to that of Pandalus danae, a shrimp that lives below the photic zone. The hypothesis was that P. resecata hemolymph would have stronger antioxidant defenses than the other crustaceans because this isopod faces a more hostile oxygen environment. LCMS analysis of P. resecata hemolymph confirmed the presence of antioxidants including pheophorbide a, lutein, and β-carotene, while L. pallasii hemolymph contained pheophorbide a and lutein but no β-carotene. Pandalus danae hemolymph had no carotenoids or pheophorbide. Although L. pallasii hemolymph was missing β-carotene, it had a significantly higher total antioxidant capacity than that of P. resecata. Hemolymph from P. danae had an intermediate antioxidant capacity even though it contained none of the antioxidants detected in the other species. The unexpected antioxidant activities among the species could be explained by differences in metabolic functions or environmental factors that were not examined in this study; or perhaps P. -

The 17Th International Colloquium on Amphipoda
Biodiversity Journal, 2017, 8 (2): 391–394 MONOGRAPH The 17th International Colloquium on Amphipoda Sabrina Lo Brutto1,2,*, Eugenia Schimmenti1 & Davide Iaciofano1 1Dept. STEBICEF, Section of Animal Biology, via Archirafi 18, Palermo, University of Palermo, Italy 2Museum of Zoology “Doderlein”, SIMUA, via Archirafi 16, University of Palermo, Italy *Corresponding author, email: [email protected] th th ABSTRACT The 17 International Colloquium on Amphipoda (17 ICA) has been organized by the University of Palermo (Sicily, Italy), and took place in Trapani, 4-7 September 2017. All the contributions have been published in the present monograph and include a wide range of topics. KEY WORDS International Colloquium on Amphipoda; ICA; Amphipoda. Received 30.04.2017; accepted 31.05.2017; printed 30.06.2017 Proceedings of the 17th International Colloquium on Amphipoda (17th ICA), September 4th-7th 2017, Trapani (Italy) The first International Colloquium on Amphi- Poland, Turkey, Norway, Brazil and Canada within poda was held in Verona in 1969, as a simple meet- the Scientific Committee: ing of specialists interested in the Systematics of Sabrina Lo Brutto (Coordinator) - University of Gammarus and Niphargus. Palermo, Italy Now, after 48 years, the Colloquium reached the Elvira De Matthaeis - University La Sapienza, 17th edition, held at the “Polo Territoriale della Italy Provincia di Trapani”, a site of the University of Felicita Scapini - University of Firenze, Italy Palermo, in Italy; and for the second time in Sicily Alberto Ugolini - University of Firenze, Italy (Lo Brutto et al., 2013). Maria Beatrice Scipione - Stazione Zoologica The Organizing and Scientific Committees were Anton Dohrn, Italy composed by people from different countries. -

Colour Polymorphism and Genetic Variation in <Emphasis Type="Italic">Idotea Baltica</Emphasis> Populations
The Ecological Distribution of British Species of Idotea (Isopoda) STOR E. Naylor The Journal of Animal Ecology, Vol. 24, No. 2. (Nov., 1955), pp. 255-269. Stable URL: http://links.jstor.org/sici?sici=0021-8790%28195511%2924%3A2%3C255%3ATEDOBS%3E2.0.CO%3B2-%23 The Journal of Animal Ecology is currently published by British Ecological Society. Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/about/terms.html. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/joumals/briteco.html. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is an independent not-for-profit organization dedicated to creating and preserving a digital archive of scholarly journals. For more information regarding JSTOR, please contact [email protected]. http://www.j stor.org/ Tue Oct 3 15:24:28 2006 VOLUME 24, No. 2 NOVEMBER 1955 THE ECOLOGICAL DISTRIBUTION OF BRITISH SPECIES OF IDOTEA (ISOPODA) BY E. NAYLOR Marine Biological Station, Port Erin (With 4 Figures in the Text) INTRODUCTION Descriptions of the ecology of Idotea are often generalized, and there appears to be no comprehensive work on the habits of individual species. -

Appendix C - Invertebrate Population Attributes
APPENDIX C - INVERTEBRATE POPULATION ATTRIBUTES C1. Taxonomic list of megabenthic invertebrate species collected C2. Percent area of megabenthic invertebrate species by subpopulation C3. Abundance of megabenthic invertebrate species by subpopulation C4. Biomass of megabenthic invertebrate species by subpopulation C- 1 C1. Taxonomic list of megabenthic invertebrate species collected on the southern California shelf and upper slope at depths of 2-476m, July-October 2003. Taxon/Species Author Common Name PORIFERA CALCEREA --SCYCETTIDA Amphoriscidae Leucilla nuttingi (Urban 1902) urn sponge HEXACTINELLIDA --HEXACTINOSA Aphrocallistidae Aphrocallistes vastus Schulze 1887 cloud sponge DEMOSPONGIAE Porifera sp SD2 "sponge" Porifera sp SD4 "sponge" Porifera sp SD5 "sponge" Porifera sp SD15 "sponge" Porifera sp SD16 "sponge" --SPIROPHORIDA Tetillidae Tetilla arb de Laubenfels 1930 gray puffball sponge --HADROMERIDA Suberitidae Suberites suberea (Johnson 1842) hermitcrab sponge Tethyidae Tethya californiana (= aurantium ) de Laubenfels 1932 orange ball sponge CNIDARIA HYDROZOA --ATHECATAE Tubulariidae Tubularia crocea (L. Agassiz 1862) pink-mouth hydroid --THECATAE Aglaopheniidae Aglaophenia sp "hydroid" Plumulariidae Plumularia sp "seabristle" Sertulariidae Abietinaria sp "hydroid" --SIPHONOPHORA Rhodaliidae Dromalia alexandri Bigelow 1911 sea dandelion ANTHOZOA --ALCYONACEA Clavulariidae Telesto californica Kükenthal 1913 "soft coral" Telesto nuttingi Kükenthal 1913 "anemone" Gorgoniidae Adelogorgia phyllosclera Bayer 1958 orange gorgonian Eugorgia -

Crustacean Fauna of a Mussel Cultivated Raft System in the Black Sea
Arthropods, 2013, 2(2): 89-94 Article Crustacean fauna of a mussel cultivated raft system in the Black Sea Murat Sezgin, Eylem Aydemir Çil Sinop University Faculty of Fisheries Department of Marine Biology and Ecology TR57000 Sinop, Turkey E-mail: [email protected] Received 1 January 2013; Accepted 5 February 2013; Published online 1 June 2013 Abstract The aim of the current study was to make a faunistic analysis of the crustaceans associated with cultivated mussels grown on ropes. Mussel samples from 30 cm ropes were collected from rope-grown mussel beds by hand. The crustacean fauna associated with mussel population were quantified. The density of crustacean fauna associated with mussels was significantly greater within rope-grown mussel assemblages than on other biotopes around. Keywords Crustacea; Mytilus galloprovincialis; raft culture; rope-grown mussels; species richness. Arthropods ISSN 22244255 URL: http://www.iaees.org/publications/journals/arthropods/onlineversion.asp RSS: http://www.iaees.org/publications/journals/arthropods/rss.xml Email: [email protected] EditorinChief: WenJun Zhang Publisher: International Academy of Ecology and Environmental Sciences 1 Introduction A few species of Bivalvia in Black Sea live on hard substrate: the mussel Mytilus galloprovincialis fasten themselves with bissal threads to underwater stones, macroalgal stems or pier columns as nature habitats. The biodiversity associated with cultivated mussel assemblages depends partly on the source of the mussels and on the new habitat created by the method of cultivation. To date, no study has been made of the macrofauna associated with mussels in the habitats created by different methods of mussel cultivation in the Black Sea. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Idotea Granulosa Rathke, 1843
Idotea granulosa Rathke, 1843 AphiaID: 119044 ISÓPODE Animalia (Reino) >Arthropoda (Filo) >Crustacea (Subfilo) >Multicrustacea (Superclasse) >Malacostraca (Classe) >Eumalacostraca (Subclasse) > Peracarida (Superordem) > Isopoda (Ordem) > Valvifera (Subordem) > Idoteidae (Familia) Rainer Borcherding - Schutzstation Wattenmeer, via beachexplorer.org Estatuto de Conservação 1 Sinónimos Idotea cretaria Dahl, 1916 Referências additional source Schotte, M., B. F. Kensley, and S. Shilling. (1995-2017). World list of Marine, Freshwater and Terrestrial Crustacea Isopoda. National Museum of Natural History Smithsonian Institution: Washington D.C., USA [website archived on 2018-01-25]. [details] additional source Rappé, G. (1989). Annoted checklist of the marine and brackish-water Isopoda (Crustacea, Malacostraca) of Belgium, in: Wouters, K.; Baert, L. (Ed.) (1989). Proceedings of the Symposium “Invertebrates of Belgium”. pp. 165-168 [details] basis of record van der Land, J. (2001). Isopoda – excluding Epicaridea, in: Costello, M.J. et al. (Ed.) (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels, 50: pp. 315-321 [details] additional source Muller, Y. (2004). Faune et flore du littoral du Nord, du Pas-de-Calais et de la Belgique: inventaire. [Coastal fauna and flora of the Nord, Pas-de-Calais and Belgium: inventory]. Commission Régionale de Biologie Région Nord Pas-de-Calais: France. 307 pp., available online at http://www.vliz.be/imisdocs/publications/145561.pdf [details] original description Rathke, H. (1843). Beiträge zur Fauna Norwegens. Nova Acta Academiae Caesareae Leopoldino-Carolinae Naturae Curiosorum, Breslau & Bonn. 20: 1-264c., available online at https://doi.org/10.5962/bhl.title.11613 [details] additional source Dyntaxa. -

Distribution and Inferred Evolutionary Characteristics of a Chimeric Ssdna Virus Associated with Intertidal Marine Isopods
Article Distribution and Inferred Evolutionary Characteristics of a Chimeric ssDNA Virus Associated with Intertidal Marine Isopods Kalia S. I. Bistolas 1,*, Ryan M. Besemer 2,3, Lars G. Rudstam 4 and Ian Hewson 1 1 Department of Microbiology, Cornell University, Ithaca, NY 14850, USA; [email protected] 2 New Visions Life Sciences, Boards of Cooperative Educational Services of New York State, Ithaca, NY 14850, USA; [email protected] 3 University of North Carolina at Wilmington, Wilmington, NC 28403, USA 4 Department of Natural Resources and the Cornell Biological Field Station, Cornell University, Bridgeport, NY 14850, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-607-255-0151 Received: 26 October 2017; Accepted: 23 November 2017; Published: 26 November 2017 Abstract: Aquatic invertebrates are common reservoirs of a rapidly expanding group of circular Rep-encoding ssDNA (CRESS-DNA) viruses. This study identified and explored the phylogenetic relationship between novel CRESS-DNA viral genotypes associated with Pacific intertidal isopods Idotea wosnesenskii, Idotea resecata, and Gnorimosphaeroma oregonensis. One genotype associated with I. wosnesenskii, IWaV278, shared sequence similarity and genomic features with Tombusviridae (ssRNA) and Circoviridae (ssDNA) genomes and was putatively assigned to the Cruciviridae clade comprising chimeric viruses. The complete genome of IWaV278 (3478 nt) was computationally completed, validated via Sanger sequencing, and exhibited sequence conservation and codon usage patterns analogous to other members of the Cruciviridae. Viral surveillance (qPCR) indicated that this virus was temporally transient (present in 2015, but not 2017), specific to I. wosnesenskii at a single collection site (Washington, DC, USA), more prevalent among male specimens, and frequently detected within exoskeletal structures. -

Invertebrate ID Guide
11/13/13 1 This book is a compilation of identification resources for invertebrates found in stomach samples. By no means is it a complete list of all possible prey types. It is simply what has been found in past ChesMMAP and NEAMAP diet studies. A copy of this document is stored in both the ChesMMAP and NEAMAP lab network drives in a folder called ID Guides, along with other useful identification keys, articles, documents, and photos. If you want to see a larger version of any of the images in this document you can simply open the file and zoom in on the picture, or you can open the original file for the photo by navigating to the appropriate subfolder within the Fisheries Gut Lab folder. Other useful links for identification: Isopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-33/htm/doc.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-48/htm/doc.html Polychaetes http://web.vims.edu/bio/benthic/polychaete.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-34/htm/doc.html Cephalopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-44/htm/doc.html Amphipods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-67/htm/doc.html Molluscs http://www.oceanica.cofc.edu/shellguide/ http://www.jaxshells.org/slife4.htm Bivalves http://www.jaxshells.org/atlanticb.htm Gastropods http://www.jaxshells.org/atlantic.htm Crustaceans http://www.jaxshells.org/slifex26.htm Echinoderms http://www.jaxshells.org/eich26.htm 2 PROTOZOA (FORAMINIFERA) ................................................................................................................................ 4 PORIFERA (SPONGES) ............................................................................................................................................... 4 CNIDARIA (JELLYFISHES, HYDROIDS, SEA ANEMONES) ............................................................................... 4 CTENOPHORA (COMB JELLIES)............................................................................................................................ -

Mediterranean Marine Science
CORE Metadata, citation and similar papers at core.ac.uk Provided by National Documentation Centre - EKT journals Mediterranean Marine Science Vol. 19, 2018 First record of the isopod Idotea hectica (Pallas, 1772) (Idoteidae) and of the brachyuran crab Matuta victor (Fabricius, 1781) (Matutidae) in the Hellenic waters KONDYLATOS Hydrobiological Station of GERASIMOS Rhodes CORSINI-FOKA MARIA Hellenic Centre for Marine Research, Hydrobiological Station of Rhodes. Cos Street, 85100 Rhodes PERAKIS EMMANOUIL Department of Fisheries Rhodes, South Aegean District. G. Mavrou 2, 85100, Rhodes https://doi.org/10.12681/mms.18106 Copyright © 2018 Mediterranean Marine Science To cite this article: KONDYLATOS, G., CORSINI-FOKA, M., & PERAKIS, E. (2018). First record of the isopod Idotea hectica (Pallas, 1772) (Idoteidae) and of the brachyuran crab Matuta victor (Fabricius, 1781) (Matutidae) in the Hellenic waters. Mediterranean Marine Science, 19(3), 656-661. doi:https://doi.org/10.12681/mms.18106 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 24/12/2020 06:42:54 | Short Communication Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.18106 First record of the isopod Idotea hectica (Pallas, 1772) (Idoteidae) and of the brachyuran crab Matuta victor (Fabricius, 1781) (Matutidae) in the Hellenic waters GERASIMOS KONDYLATOS1, MARIA CORSINI-FOKA1 and EMMANOUIL PERAKIS2 1 Hellenic Centre for Marine Research, -

The Growth, Reproduction and Body Color Pattern of Cleantiella Isopus (Isopoda: Valvifera) in Hakodate Bay, Japan
CRUSTACEAN RESEARCH, NO. 41: 1–10, 2012 The growth, reproduction and body color pattern of Cleantiella isopus (Isopoda: Valvifera) in Hakodate Bay, Japan Tomohiro Takahashi and Seiji Goshima A b s t r a c t . — We studied the growth, cycling in the intertidal and subtidal areas. reproduction and body color pattern of the Most isopod studies focus on the life marine isopod Cleantiella isopus in Hakodate Bay, cycle, feeding habits and mate choice; Japan from May 2009 to July 2010. Individuals almost all are exclusively on European were collected every month and the sex, body species of the genus Idotea (Naylor, 1955a, length, color pattern, number of eggs per clutch b; Jormalainen et al., 1992). The breeding and developmental stage of embryos for ovigerous period and its length differ among species females were recorded. Five body color patterns or conspecific populations that occupy were identified in C. isopus at Hakodate Bay, and different habitats (Naylor, 1955b; Sheader, their composition was maintained throughout 1977; Salemaa, 1979; Healy & O’Neill, the year. Breeding females (guarded by a male 1984). Although about 20 species have been or carrying eggs) were observed in the field from reported in Japan, as far as we know, there February to August. Newly recruited individuals is only one study about the feeding habit of were first observed in July and grew to mature size by the next breeding season, indicating a Idotea ochotensis (Suzuki et al., 2002). Isopod lifespan of 13–15 months. Precopulatory mate species are common in northern Japan and guarding in which a male holds a female using his play an important role in the community of pereopods was observed. -
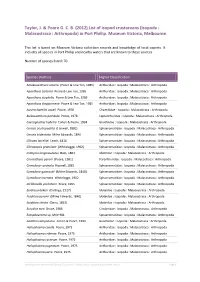
List of Isopod Crustaceans (Isopoda : Malacostraca : Arthropoda) in Port Phillip
Taylor, J. & Poore G. C. B. (2012) List of isopod crustaceans (Isopoda : Malacostraca : Arthropoda) in Port Phillip. Museum Victoria, Melbourne. This list is based on Museum Victoria collection records and knowledge of local experts. It includes all species in Port Phillip and nearby waters that are known to these sources. Number of species listed: 70. Species (Author) Higher Classification Amakusanthura olearia (Poore & Lew Ton, 1985) Anthuridae : Isopoda : Malacostraca : Arthropoda Apanthura isotoma Poore & Lew Ton, 1985 Anthuridae : Isopoda : Malacostraca : Arthropoda Apanthura styphelia Poore & Lew Ton, 1985 Anthuridae : Isopoda : Malacostraca : Arthropoda Apanthura thryptomene Poore & Lew Ton, 1985 Anthuridae : Isopoda : Malacostraca : Arthropoda Austrochaetilia capeli Poore, 1978 Chaetiliidae : Isopoda : Malacostraca : Arthropoda Bullowanthura pambula Poore, 1978 Leptanthuridae : Isopoda : Malacostraca : Arthropoda Caecognathia huberia Cohen & Poore, 1994 Gnathiidae : Isopoda : Malacostraca : Arthropoda Cerceis acuticaudata (Haswell, 1881) Sphaeromatidae : Isopoda : Malacostraca : Arthropoda Cerceis tridentata Milne Edwards, 1840 Sphaeromatidae : Isopoda : Malacostraca : Arthropoda Cilicaea latreillei Leach, 1818 Sphaeromatidae : Isopoda : Malacostraca : Arthropoda Cilicaeopsis granulata (Whitelegge, 1902) Sphaeromatidae : Isopoda : Malacostraca : Arthropoda Crabyzos longicaudatus Bate, 1863 Idoteidae : Isopoda : Malacostraca : Arthropoda Cruranthura peroni (Poore, 1981) Paranthuridae : Isopoda : Malacostraca : Arthropoda Cymodoce