A Pollen Rain-Vegetation Study Along a 3600 M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caryophyllales 2018 Instituto De Biología, UNAM September 17-23
Caryophyllales 2018 Instituto de Biología, UNAM September 17-23 LOCAL ORGANIZERS Hilda Flores-Olvera, Salvador Arias and Helga Ochoterena, IBUNAM ORGANIZING COMMITTEE Walter G. Berendsohn and Sabine von Mering, BGBM, Berlin, Germany Patricia Hernández-Ledesma, INECOL-Unidad Pátzcuaro, México Gilberto Ocampo, Universidad Autónoma de Aguascalientes, México Ivonne Sánchez del Pino, CICY, Centro de Investigación Científica de Yucatán, Mérida, Yucatán, México SCIENTIFIC COMMITTEE Thomas Borsch, BGBM, Germany Fernando O. Zuloaga, Instituto de Botánica Darwinion, Argentina Victor Sánchez Cordero, IBUNAM, México Cornelia Klak, Bolus Herbarium, Department of Biological Sciences, University of Cape Town, South Africa Hossein Akhani, Department of Plant Sciences, School of Biology, College of Science, University of Tehran, Iran Alexander P. Sukhorukov, Moscow State University, Russia Michael J. Moore, Oberlin College, USA Compilation: Helga Ochoterena / Graphic Design: Julio C. Montero, Diana Martínez GENERAL PROGRAM . 4 MONDAY Monday’s Program . 7 Monday’s Abstracts . 9 TUESDAY Tuesday ‘s Program . 16 Tuesday’s Abstracts . 19 WEDNESDAY Wednesday’s Program . 32 Wednesday’s Abstracs . 35 POSTERS Posters’ Abstracts . 47 WORKSHOPS Workshop 1 . 61 Workshop 2 . 62 PARTICIPANTS . 63 GENERAL INFORMATION . 66 4 Caryophyllales 2018 Caryophyllales General program Monday 17 Tuesday 18 Wednesday 19 Thursday 20 Friday 21 Saturday 22 Sunday 23 Workshop 1 Workshop 2 9:00-10:00 Key note talks Walter G. Michael J. Moore, Berendsohn, Sabine Ya Yang, Diego F. Registration -
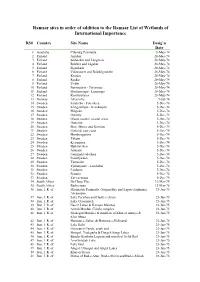
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

LCSH Section K
K., Rupert (Fictitious character) Motion of K stars in line of sight Ka-đai language USE Rupert (Fictitious character : Laporte) Radial velocity of K stars USE Kadai languages K-4 PRR 1361 (Steam locomotive) — Orbits Ka’do Herdé language USE 1361 K4 (Steam locomotive) UF Galactic orbits of K stars USE Herdé language K-9 (Fictitious character) (Not Subd Geog) K stars—Galactic orbits Ka’do Pévé language UF K-Nine (Fictitious character) BT Orbits USE Pévé language K9 (Fictitious character) — Radial velocity Ka Dwo (Asian people) K 37 (Military aircraft) USE K stars—Motion in line of sight USE Kadu (Asian people) USE Junkers K 37 (Military aircraft) — Spectra Ka-Ga-Nga script (May Subd Geog) K 98 k (Rifle) K Street (Sacramento, Calif.) UF Script, Ka-Ga-Nga USE Mauser K98k rifle This heading is not valid for use as a geographic BT Inscriptions, Malayan K.A.L. Flight 007 Incident, 1983 subdivision. Ka-houk (Wash.) USE Korean Air Lines Incident, 1983 BT Streets—California USE Ozette Lake (Wash.) K.A. Lind Honorary Award K-T boundary Ka Iwi National Scenic Shoreline (Hawaii) USE Moderna museets vänners skulpturpris USE Cretaceous-Paleogene boundary UF Ka Iwi Scenic Shoreline Park (Hawaii) K.A. Linds hederspris K-T Extinction Ka Iwi Shoreline (Hawaii) USE Moderna museets vänners skulpturpris USE Cretaceous-Paleogene Extinction BT National parks and reserves—Hawaii K-ABC (Intelligence test) K-T Mass Extinction Ka Iwi Scenic Shoreline Park (Hawaii) USE Kaufman Assessment Battery for Children USE Cretaceous-Paleogene Extinction USE Ka Iwi National Scenic Shoreline (Hawaii) K-B Bridge (Palau) K-TEA (Achievement test) Ka Iwi Shoreline (Hawaii) USE Koro-Babeldaod Bridge (Palau) USE Kaufman Test of Educational Achievement USE Ka Iwi National Scenic Shoreline (Hawaii) K-BIT (Intelligence test) K-theory Ka-ju-ken-bo USE Kaufman Brief Intelligence Test [QA612.33] USE Kajukenbo K. -

EPRA International Journal of Research and Development (IJRD) Volume: 5 | Issue: 12 | December 2020 - Peer Reviewed Journal
SJIF Impact Factor: 7.001| ISI I.F.Value:1.241| Journal DOI: 10.36713/epra2016 ISSN: 2455-7838(Online) EPRA International Journal of Research and Development (IJRD) Volume: 5 | Issue: 12 | December 2020 - Peer Reviewed Journal CHEMICAL EVIDENCE SUPPORTING THE ICLUSION OF AMARANTHACEAE AND CHENOPODIACEAE INTO ONE FAMILY AMARANTHACEAE JUSS. (s.l.) Fatima Mubark1 1PhD Research Scholar, Medicinal and Aromatic Plants research Institute, National Council for Research, Khartom, Sudan Ikram Madani Ahmed2 2Associate Professor, Department of Botany, Faculty of Science, University of Khartoum, Sudan Corresponding author: Ikram Madani, Article DOI: https://doi.org/10.36713/epra6001 ABSTRACT In this study, separation of chemical compounds using Thin layer chromatography technique revealed close relationship between the studied members of the newly constructed family Amaranthaceae Juss. (s.l.). 68% of the calculated affinities between the studied species are above 50% which is an indication for close relationships. 90% is the chemical affinities reported between Chenopodium murale and three species of the genus Amaranthus despite of their great morphological diversity. Among the selected members of the chenopodiaceae, Chenopodium murale and Suaeda monoica are the most closely related species to all of the studied Amaranthaceae . 60%-88% and 54%-88% chemical affinities were reported for the two species with the Amaranthaceae members respectively. GC-Mass analysis of methanolic extracts of the studied species identified 20 compounds common between different species. 9,12- Octadecadienoic acid (Z,Z)-,2-hydroxy-1 and 7-Hexadecenal,(Z)- are the major components common between Amaranthus graecizans, Digera muricata Aerva javanica Gomphrena celosioides of the historical family Amaranthaceae and Suaeda monoica Salsola vermiculata Chenopodium murale Cornulaca monocantha of the historical family Chenopodiaceae, Most of the identified compounds are of pharmaceutical importance such as antioxidants, anti-inflammatory , and Anti-cancerous. -

Considerations About Semitic Etyma in De Vaan's Latin Etymological Dictionary
applyparastyle “fig//caption/p[1]” parastyle “FigCapt” Philology, vol. 4/2018/2019, pp. 35–156 © 2019 Ephraim Nissan - DOI https://doi.org/10.3726/PHIL042019.2 2019 Considerations about Semitic Etyma in de Vaan’s Latin Etymological Dictionary: Terms for Plants, 4 Domestic Animals, Tools or Vessels Ephraim Nissan 00 35 Abstract In this long study, our point of departure is particular entries in Michiel de Vaan’s Latin Etymological Dictionary (2008). We are interested in possibly Semitic etyma. Among 156 the other things, we consider controversies not just concerning individual etymologies, but also concerning approaches. We provide a detailed discussion of names for plants, but we also consider names for domestic animals. 2018/2019 Keywords Latin etymologies, Historical linguistics, Semitic loanwords in antiquity, Botany, Zoonyms, Controversies. Contents Considerations about Semitic Etyma in de Vaan’s 1. Introduction Latin Etymological Dictionary: Terms for Plants, Domestic Animals, Tools or Vessels 35 In his article “Il problema dei semitismi antichi nel latino”, Paolo Martino Ephraim Nissan 35 (1993) at the very beginning lamented the neglect of Semitic etymolo- gies for Archaic and Classical Latin; as opposed to survivals from a sub- strate and to terms of Etruscan, Italic, Greek, Celtic origin, when it comes to loanwords of certain direct Semitic origin in Latin, Martino remarked, such loanwords have been only admitted in a surprisingly exiguous num- ber of cases, when they were not met with outright rejection, as though they merely were fanciful constructs:1 In seguito alle recenti acquisizioni archeologiche ed epigrafiche che hanno documen- tato una densità finora insospettata di contatti tra Semiti (soprattutto Fenici, Aramei e 1 If one thinks what one could come across in the 1890s (see below), fanciful constructs were not a rarity. -

Senecio Glaucus Subsp. Coronopifolius ) (MAIRE) C
Az. J. Pharm Sci. Vol. 52, September, 2015. 283 PHYTOCHEMICAL AND BIOLOGICAL STUDY OF (Senecio glaucus subsp. coronopifolius ) (MAIRE) C. ALEXANDER GROWING IN EGYPT BY Shaza A. Mohamed FROM Pharmacognosy Department, Faculty of Pharmacy (Girls), AL-Azhar University, Cairo, Egypt. ABSTRACT Senecio glaucus subsp. coronopifolius (Maire) C. Alexander is wild annual herb distributed in the Egyptian deserts. Total phenolic and flavonoid content of plant root were determined using both HPLC and colorimetric analysis. Syringic acid and hesperidin (1378.802 and 6638.247 mg / 100 gm. dried plant root powder, respectively) were of the highest concentration compounds resulted from HPLC analysis of total phenolic and flavonoid content. The colorimetric estimation of total phenolic and flavonoid content resulted in concentration of (98.23 ± 0.28 mg/gm. expressed as Gallic acid equivalent (GAE) and 35.9± 0.17 mg/gm. expressed as quercetin equivalent (QE), respectively). GC-MS analysis of un-saponifiable matters and fatty acid methyl esters of the plant leaves indicated that octacosane (11.85%) and linolenic acid methyl ester (31.07%) (poly- unsaturated fatty acid) were the major identified compounds, respectively. The DNA of the plant was analyzed using twelve random decamer primers. A total of 52 random amplified polymorphic DNA (RAPD) markers were identified. Root extracts (ethyl acetate, acetone and methyl alcohol) were subjected to determine the antimicrobial behavior and also their cytotoxic activity, by using (3- (4, 5- dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) (MTT) assay against colon carcinoma cell lines (HCT-116). Among the fore mentioned extracts, root ethyl acetate extract gave appreciable antibacterial and antifungal behavior and also had promising cytotoxic activity with IC50 = 7.39 ±1.2 µg/ml. -

CHAPTER 3 HYDROLOGICAL MODELING 3.1 Introduction 3.2
The Study on Flood and Debris Flow Supporting Report I (Master Plan) Paper IV in the Caspian Coastal Area Meteo-Hydrology focusing on the Flood-hit Region in Golestan Province CHAPTER 3 HYDROLOGICAL MODELING 3.1 Introduction An integrated and distributed MIKE SHE hydrological model is used to evaluate rainfall-runoff process in the Madarsoo River basin. The model is able to analyze impacts of watershed management practices, land use, soil types, topographic features, flow regulation structures, etc. over the basin on river flows. For this, MIKE SHE model was coupled with MIKE 11 river modeling system to simulate flows in the river system. Inflows and hydrodynamic processes in rivers are taken into consideration for model development. The model computes river flows taking account of overland flow, interflow and base-flow. An integrated and distributed hydrological model MIKE SHE was set up for the Madarsoo River basin for the following reasons: (1) To generate probable or design discharges precisely in the river system to assist on flood control master plan development, (2) To analyze the impacts of watershed management practices and biological measures of flood mitigation by quantifying river flows under these circumstances, and (3) To analyze the impact of incorporation of flood regulation structures like dam in the river system to reduce peak flows. 3.2 MIKE SHE MIKE SHE is an integrated hydrological model because all components of hydrological cycle (precipitation, evapotranspiration, surface flow, infiltration, groundwater flow, etc.) are incorporated into the model. Similarly, and it is also a distributed model because the model can handle spatial and temporal distributions of parameters. -

Guidance Document Pohakuloa Training Area Plant Guide
GUIDANCE DOCUMENT Recovery of Native Plant Communities and Ecological Processes Following Removal of Non-native, Invasive Ungulates from Pacific Island Forests Pohakuloa Training Area Plant Guide SERDP Project RC-2433 JULY 2018 Creighton Litton Rebecca Cole University of Hawaii at Manoa Distribution Statement A Page Intentionally Left Blank This report was prepared under contract to the Department of Defense Strategic Environmental Research and Development Program (SERDP). The publication of this report does not indicate endorsement by the Department of Defense, nor should the contents be construed as reflecting the official policy or position of the Department of Defense. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the Department of Defense. Page Intentionally Left Blank 47 Page Intentionally Left Blank 1. Ferns & Fern Allies Order: Polypodiales Family: Aspleniaceae (Spleenworts) Asplenium peruvianum var. insulare – fragile fern (Endangered) Delicate ENDEMIC plants usually growing in cracks or caves; largest pinnae usually <6mm long, tips blunt, uniform in shape, shallowly lobed, 2-5 lobes on acroscopic side. Fewer than 5 sori per pinna. Fronds with distal stipes, proximal rachises ocassionally proliferous . d b a Asplenium trichomanes subsp. densum – ‘oāli’i; maidenhair spleenwort Plants small, commonly growing in full sunlight. Rhizomes short, erect, retaining many dark brown, shiny old stipe bases.. Stipes wiry, dark brown – black, up to 10cm, shiny, glabrous, adaxial surface flat, with 2 greenish ridges on either side. Pinnae 15-45 pairs, almost sessile, alternate, ovate to round, basal pinnae smaller and more widely spaced. -

CHENOPODIACEAE 藜科 Li Ke Zhu Gelin (朱格麟 Chu Ge-Ling)1; Sergei L
Flora of China 5: 351-414. 2003. CHENOPODIACEAE 藜科 li ke Zhu Gelin (朱格麟 Chu Ge-ling)1; Sergei L. Mosyakin2, Steven E. Clemants3 Herbs annual, subshrubs, or shrubs, rarely perennial herbs or small trees. Stems and branches sometimes jointed (articulate); indumentum of vesicular hairs (furfuraceous or farinose), ramified (dendroid), stellate, rarely of glandular hairs, or plants glabrous. Leaves alternate or opposite, exstipulate, petiolate or sessile; leaf blade flattened, terete, semiterete, or in some species reduced to scales. Flowers monochlamydeous, bisexual or unisexual (plants monoecious or dioecious, rarely polygamous); bracteate or ebracteate. Bractlets (if present) 1 or 2, lanceolate, navicular, or scale-like. Perianth membranous, herbaceous, or succulent, (1–)3–5- parted; segments imbricate, rarely in 2 series, often enlarged and hardened in fruit, or with winged, acicular, or tuberculate appendages abaxially, seldom unmodified (in tribe Atripliceae female flowers without or with poorly developed perianth borne between 2 specialized bracts or at base of a bract). Stamens shorter than or equaling perianth segments and arranged opposite them; filaments subulate or linear, united at base and usually forming a hypogynous disk, sometimes with interstaminal lobes; anthers dorsifixed, incumbent in bud, 2-locular, extrorse, or dehiscent by lateral, longitudinal slits, obtuse or appendaged at apex. Ovary superior, ovoid or globose, of 2–5 carpels, unilocular; ovule 1, campylotropous; style terminal, usually short, with 2(–5) filiform or subulate stigmas, rarely capitate, papillose, or hairy on one side or throughout. Fruit a utricle, rarely a pyxidium (dehiscent capsule); pericarp membranous, leathery, or fleshy, adnate or appressed to seed. Seed horizontal, vertical, or oblique, compressed globose, lenticular, reniform, or obliquely ovoid; testa crustaceous, leathery, membranous, or succulent; embryo annular, semi-annular, or spiral, with narrow cotyledons; endosperm much reduced or absent; perisperm abundant or absent. -

Central Asia
#1 Central Asia Snow leopard. All three big cats in the region – Persian leopard, Asiatic cheetah and snow leopard – are threatened by illegal hunting. Hunting of the cats' natural prey also causes starvation and increases the likelihood of attacks on domestic animals. 14 | | 15 Contents #1 3 _ Ongoing conservation efforts 54 List of figures 18 List of tables 18 3.1 Government 56 List of boxes 18 3.1.1 Institutions for conservation 56 List of abbreviations and acronyms 18 3.1.2 Protected areas 59 3.1.3 Transboundary initiatives 60 3.1.4 Wildlife law enforcement 62 3.1.5 National and local policies 63 0 _ Executive summary 20 3.1.6 International agreements 66 3.2 Community-based conservation 67 3.3 Civil society 67 1 _ Background 24 3.3.1 CSOs in Central Asia 67 3.3.2 CSO/NGO approaches and projects 68 1.1 Socio-economic setting 26 3.4 Private sector 72 1.1.1 Political and administrative context 26 3.5 International agencies and donors 73 1.1.2 Population and livelihoods 27 1.1.3 Economy 29 1.1.4 Resource ownership and governance 30 1.2 Key biodiversity features 31 4 _ Lessons learned 78 1.2.1 Geography and climate 31 4.1 Protected areas 80 1.2.2 Habitats and ecosystems 32 4.2 Landscape approaches to conservation 81 1.2.3 Species diversity, endemicity and extinction risk 35 4.3 Transboundary initiatives 82 1.2.4 Geographic priorities for conservation 36 4.4 Wildlife crime 82 4.5 Trophy and market hunting 84 4.6 Civil society organisations 85 2 _ Conservation challenges 40 4.7 Biodiversity conservation research 85 4.8 Private sector 85 -

Chromosome Counts of Some Angiosperm Species from Iran
NEW OR RARE CHROMOSOME COUNTS OF SOME ANGIOSPERM SPECIES FROM IRAN S. M. GHAFFARI Ghaffari, S. M. 2006 01 01: New or rare chromosome counts of some angiosperm species from Iran. –Iran. Journ. Bot. 11 (2): 185-192. Tehran. Original chromosome observations including 29 species from 19 families are reported. Of these, the chromosome numbers, for 6 taxa including, Cirsium hygrophilum (Asteraceae), Matthiola longipetala subsp. bicornis (Brassicaceae), Dianthus orientalis subsp. nassireddini (Caryophyllaceae), Phlomis bruguieri, Salvia leriifolia, Teucrium oliverianum (Lamiaceae) are new observations. Also, new tetraploid level of n=24 for Asyneuma amplexicaule (Campanulaceae) and new diploid level of n=9 for Euphorbia microsciadia (Euphorbiaceae) are reported here for the first time. Seyed Mahmood Ghaffari, Institute of Biochemistry and Biophysics, University of Tehran. P. O. Box 13145-1384, Tehran, Iran. E-mail: [email protected] Keywords. Chromosomes, Angiospermae, Iran. ﺷﻤﺎرش ﮐﺮوﻣﻮزوﻣﯽ ﺟﺪﯾﺪ ﯾﺎ ﻧﺎدر ﺑﺮاي ﺑﻌﻀﯽ از ﮔﻮﻧﻪﻫﺎي ﻧﻬﺎﻧﺪاﻧﻪ اﯾﺮان ﺳﯿﺪﻣﺤﻤﻮد ﻏﻔﺎري ﻣﺸﺎﻫﺪات ﮐﺮوﻣﻮزوﻣﯽ 29 ﮔﻮﻧﻪ ﻣﺘﻌﻠﻖ ﺑﻪ 19 ﺧﺎﻧﻮاده ﮔﺰارش ﻣﯽﺷﻮد. ﺷﻤﺎرﺷﻬﺎي ﮐﺮوﻣﻮزوﻣﯽ ﺑﺮاي 6 ﺗﺎﮐﺴﻮن ﺷﺎﻣﻞ: Cirsium hygrophilum (Asteraceae), Matthiola longipetala subsp. bicornis (Brassicaceae), Dianthus orientalis subsp. Nassireddini (Caryophyllaceae), Phlomis bruguieri, Salvia leriifolia, Teucrium (oliverianum (Lamiaceae ﺟﺪﯾﺪ ﻣﯽﺑﺎﺷﻨﺪ. ﻫﻤﭽﻨﯿﻦ ﺳﻄﺢ ﺗﺘﺮاﭘﻠﻮﺋﯿﺪي n=24 ﺑﺮاي ﮔﻮﻧﻪ (Asyneuma amplexicaule (Campanulaceae و ﺳﻄﺢ دﯾﭙﻠﻮﺋﯿﺪي n=9 ﺑﺮاي ﮔﻮﻧﻪ (Euphorbia microsciadia (Euphorbiaceae ﺑﺮاي اوﻟﯿﻦ ﺑﺎر ﮔﺰارش ﻣﯽﺷﻮد. 186 S. M. Ghaffari IRAN. JOURN. BOT. 11 (2), 2006 INTRODUCTION Tehran University (TUH) or in the IRAN The purpose of this paper and others forth Herbarium (Ghaffari, 1986, 1987a, 1987b, 1988) coming in the same series, is to give information RESULTS AND DISCUSSION concerning the chromosome counts of Angiosperm taxa of the Iranian flora. -

December 2020 Volume 45, Issue 12 the Men’S Garden Club of Burlington NC
WE HELP GARDENERS GROW The Seedling — December 2020 Volume 45, Issue 12 The Men’s Garden Club of Burlington NC https://burlingtonmensgarden.club Next Meeting: January 26, 2021 Bring a guest! NO MEETING IN DECEMBER Topic: No Meeting Send suggestions for 2021 programs A Message from President Dirk Sprenger Dear Members and Friends of the passed away. Both were very active Garden Club in this club and we miss them. We achieved many goals. Visitors came Inside this issue: I love December and reading to some of the Zoom meetings. We about Holiday plants. We have learned had some excellent speakers from Editor’s Corner 2 so much about milkweed in recent outside the club. We raised as much weeks. Not a holiday plant, but it is money as we did the year before. Member Birthdays 2 endangered. My belief is that it is not a We enjoyed our first November Upcoming meeting 2 weed at all, but was misnamed by picnic and learned how to use our Club News 3 pioneers who tried to make a casserole gardening skills to grow more food. with it. The monarch butterfly needs it We shared seeds and apple-wood. In Memoriam: John 3 as the staff of life. We need each other Learning the time to get orchids on Black and our last two meetings were sale and how to keep them alive was amazing. a real plus in my book. Thanks Ray! Zoom Meeting 4 My thanks go to Harry for the We canceled the plans to sell Gardening in the 5 program in November about butterflies plants at the Dogwood Festival very and milkweed.