Exploring Riparian Vegetation Interactions with Flow Regime and Fluvial Processes for an Improved River Management and Conservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Switzerland - Alpine Flowers of the Upper Engadine
Switzerland - Alpine Flowers of the Upper Engadine Naturetrek Tour Report 8 - 15 July 2018 Androsace alpina Campanula cochlerariifolia The group at Piz Palu Papaver aurantiacum Report and Images by David Tattersfield Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Switzerland - Alpine Flowers of the Upper Engadine Tour participants: David Tattersfield (leader) with 16 Naturetrek clients Day 1 Sunday 8th July After assembling at Zurich airport, we caught the train to Zurich main station. Once on the intercity express, we settled down to a comfortable journey, through the Swiss countryside, towards the Alps. We passed Lake Zurich and the Walensee, meeting the Rhine as it flows into Liectenstein, and then changed to the UNESCO World Heritage Albula railway at Chur. Dramatic scenery and many loops, tunnels and bridges followed, as we made our way through the Alps. After passing through the long Preda tunnel, we entered a sunny Engadine and made a third change, at Samedan, for the short ride to Pontresina. We transferred to the hotel by minibus and met the remaining two members of our group, before enjoying a lovely evening meal. After a brief talk about the plans for the week, we retired to bed. Day 2 Monday 9th July After a 20-minute walk from the hotel, we caught the 9.06am train at Surovas. We had a scenic introduction to the geography of the region, as we travelled south along the length of Val Bernina, crossing the watershed beside Lago Bianco and alighting at Alp Grum. -

Cross Taxon Congruence Between Lichens and Vascular Plants in a Riparian Ecosystem
diversity Article Cross Taxon Congruence Between Lichens and Vascular Plants in a Riparian Ecosystem Giovanni Bacaro 1,* , Enrico Tordoni 1 , Stefano Martellos 1 , Simona Maccherini 2 , Michela Marignani 3 , Lucia Muggia 1 , Francesco Petruzzellis 1 , Rossella Napolitano 1, Daniele Da Re 4, Tommaso Guidi 5, Renato Benesperi 5 , Vincenzo Gonnelli 6 and Lorenzo Lastrucci 7 1 Department of Life Sciences, University of Trieste, Via L. Giorgieri 10, 34127 Trieste, Italy 2 Department of Life Sciences, University of Siena, Via P.A. Mattioli 4, 53100 Siena, Italy 3 Department of Life and Environmental Sciences, Botany Division, University of Cagliari, Viale S. Ignazio, 13, 09123 Cagliari, Italy 4 Georges Lemaître Institute for Earth and Climate Research, Université catholique de Louvain, Place Louis Pasteur 3, 1348 Louvain-la-Neuve, Belgium 5 Department of Biology, University of Florence, Via G. La Pira 4, 50121 Florence, Italy 6 Istituto di Istruzione Superiore “Camaiti”, Via San Lorenzo 18, 52036 Pieve Santo Stefano, Arezzo, Italy 7 University Museum System, Natural History Museum of the University of Florence, Botany, Via La Pira 4, 50121 Florence, Italy * Correspondence: [email protected]; Tel.: +39-040-5588803 Received: 23 February 2019; Accepted: 6 August 2019; Published: 13 August 2019 Abstract: Despite that congruence across taxa has been proved as an effective tool to provide insights into the processes structuring the spatial distribution of taxonomic groups and is useful for conservation purposes, only a few studies on cross-taxon congruence focused on freshwater ecosystems and on the relations among vascular plants and lichens. We hypothesized here that, since vascular plants could be good surrogates of lichens in these ecosystems, it would be possible to assess the overall biodiversity of riparian habitats using plant data only. -

Riparian Vegetation of the Iberian Peninsula. Composition and Structure
Riparian vegetation of the Iberian Peninsula. Composition and structure. Riparian Vegetation of the Iberian Peninsula. Composition and structure. Ignacio García-Amorena, Juan Manuel Rubiales Jiménez E.T.S. Ingenieros de Montes (U. D. Botánica). Universidad Politécnica de Madrid. 1. Introduction The riparian vegetation can be defined as the vegetation influenced by the existence of the river. In flat areas it can expand kilometres away from the channel while in steeper areas can stick to a few meters from it. In comparison with the surrounding areas, the riparian environment can be characterised by the following features: 9 Higher water availability 9 Higher environmental humidity 9 Less severe temperature extremes as a result of water and vegetation smoother effect. 9 Soil dependency on fluvial regime (migration or sedimentation of different materials, erosion…) Ideal vegetal distribution along the river follows two different axes: 1. Longitudinal (following the river line): In response to the altitudinal gradient, species distribute according to their optimum climatic conditions. The slope of the river line and the fluvial regime (frequency of floodings, runoffs and drought periods) varies with the altitude (Fig. 1), conditioning the species distribution. Fig. 1. Longitudinal section of a typical short mountain river. 2. Transverse section: Species distribution depends on the distance of the surface to the phreatic water table, therefore to the distance to the channel (the topography takes here a lot of importance conditioning the extension of the riparian vegetation). Accordingly, the following zones can be distinguished: 9 River line; where aquatic and first line phreatic plants can be found. 9 Hydrologic floodplain; home of the second line of phreatic plants. -

Quarterly Changes
Plant Names Database: Quarterly changes 1 March 2020 © Landcare Research New Zealand Limited 2020 This copyright work is licensed under the Creative Commons Attribution 4.0 license. Attribution if redistributing to the public without adaptation: "Source: Landcare Research" Attribution if making an adaptation or derivative work: "Sourced from Landcare Research" http://dx.doi.org/10.26065/d37z-6s65 CATALOGUING IN PUBLICATION Plant names database: quarterly changes [electronic resource]. – [Lincoln, Canterbury, New Zealand] : Landcare Research Manaaki Whenua, 2014- . Online resource Quarterly November 2014- ISSN 2382-2341 I.Manaaki Whenua-Landcare Research New Zealand Ltd. II. Allan Herbarium. Citation and Authorship Wilton, A.D.; Schönberger, I.; Gibb, E.S.; Boardman, K.F.; Breitwieser, I.; Cochrane, M.; de Pauw, B.; Ford, K.A.; Glenny, D.S.; Korver, M.A.; Novis, P.M.; Prebble J.; Redmond, D.N.; Smissen, R.D. Tawiri, K. (2020) Plant Names Database: Quarterly changes. March 2020. Lincoln, Manaaki Whenua Press. This report is generated using an automated system and is therefore authored by the staff at the Allan Herbarium who currently contribute directly to the development and maintenance of the Plant Names Database. Authors are listed alphabetically after the third author. Authors have contributed as follows: Leadership: Wilton, Schönberger, Breitwieser, Smissen Database editors: Wilton, Schönberger, Gibb Taxonomic and nomenclature research and review: Schönberger, Gibb, Wilton, Breitwieser, Ford, Glenny, Novis, Redmond, Smissen Information System development: Wilton, De Pauw, Cochrane Technical support: Boardman, Korver, Redmond, Tawiri Disclaimer The Plant Names Database is being updated every working day. We welcome suggestions for improvements, concerns, or any data errors you may find. Please email these to [email protected]. -

Czech Species of the Gall-Making Sawflies of the Genera Phyllocolpa, Tubpontania and Pontania (Hymenoptera, Nematinae)
ISSN 1211-8788 Acta Musei Moraviae, Scientiae biologicae (Brno) 100(1): 137–156, 2015 Czech species of the gall-making sawflies of the genera Phyllocolpa, Tubpontania and Pontania (Hymenoptera, Nematinae) KAREL BENEŠ Kreuzmannova 14, CZ-318 00 Plzeò, Czech Republic; e-mail: [email protected] BENEŠ K. 2015: Czech species of the gall-making sawflies of the genera Phyllocolpa, Tubpontania and Pontania (Hymenoptera, Tenthredinidae). Acta Musei Moraviae, Scientiae biologicae (Brno) 100(1): 137–156. – Galls of the nematine genera Phyllocolpa Benson, 1960, Tubpontania Vikberg, 2010, and Pontania Costa, 1852 from the Prof. Baudyš herbarium and the author´s own records were revised and recorded in terms of recent taxonomic developments. The following species and subspecies have thus been recorded as new to the Czech species list: Ph. plicadaphnoides Kopelke, 2007, Ph. polita (Zaddach, 1883), Ph. prussica (Zaddach, 1883), Ph. rolleri Liston, 2005, T. cyrnea (Liston, 2005), P. (E.) acufoliae acutifoliae Zinovjev, 1985 and P. (E.) collactanea rosmarinifoliae Vikberg et Zinovjev, 2006. Key words. Phyllocolpa, Tubpontania, Pontania, new records, galls, distribution, key Introduction The first contributions to knowledge of sawfly galls were published about in the early-to-mid 20th century in a number of papers by BAUDYŠ (e.g. 1915, 1916, 1922, 1924, 1925 1926a, b, 1943–4, 1948, 1953, 1954) and BAYER (e. g. 1914, 1928). The first review of species of the subfamily Nematinae known from the Czech Lands was published by GREGOr & BAŤA (1942), who recorded 13 species of the genus Pontania. Five of them are now classified as Phyllocolpa, and seven as Pontania. P. fibulata Konow, 1901 has been synonymized with Ph. -

Coriaria Myrtifolia-Dominated Vegetation: Syntaxonomic Considerations on a Newly Found Community Type in Tuscany (Italy)
Plant Sociology, Vol. 56, No. 2, December 2019, pp. 99-112 DOI 10.7338/pls2019562/07 Coriaria myrtifolia-dominated vegetation: syntaxonomic considerations on a newly found community type in Tuscany (Italy) G. Bonari1, T. Fiaschi2, K. Chytrý1, M. Biagioli3, C. Angiolini2 1Department of Botany and Zoology, Masaryk University, Kotlářská 2, CZ-611 37 Brno, Czech Republic. 2Department of Life Sciences, University of Siena, Via P.A. Mattioli 4, I-53018, Siena, Italy. 3Meteo Siena 24, Via Piave 2, I-53100 Siena, Italy. Gianmaria Bonari https://orcid.org/0000-0002-5574-6067, Tiberio Fiaschi https://orcid.org/0000-0003-0403-2387, Kryštof Chytrý https://orcid.org/0000-0003-4113-6564, Marco Biagioli, Claudia Angiolini https://orcid.org/0000- 0002-9125-764X Abstract During botanical researches, we found an isolated population of Coriaria myrtifolia for the first time in Tuscany (Italy). This study aims to gain insights into the distribution of this species and its associated vegetation. We studied the scrub vegetation dominated by C. myrtifolia at the currently known southernmost limit of its distribution in Italy through the phytosociological method. We present and discuss the attribution of the Tuscan relevés to the association Rubo ulmifolii-Coriarietum myrtifoliae O. de Bolòs 1954 (Pruno spinosae-Rubion ulmifolii O. de Bolòs 1954), firstly reported for peninsular Italy. Our data allowed us to describe a new subassociation viburnetosum tini differentiated by the Mediterranean shrub Viburnum tinus subsp. tinus and by the meso-xerophilous herbs Lathyrus latifolius and Viola alba subsp. dehnhardtii. This research also suggests that, although vast areas of Tuscany lie in the Temperate submediterranean macrobioclimate, including our study area, the presence of Mediterranean elements in the shrub vegetation can be conspicuous when local factors, such as a water body, mitigate the microclimate. -
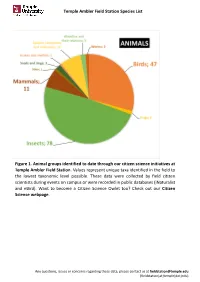
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

Copertina in A4
ISSN 2532-8034 (Online) Notiziario della Società Botanica VOL. 3(1) 2019 Italiana Notiziario della Società Botanica Italiana rivista online http://notiziario.societabotanicaitaliana.it pubblicazione semestrale decreto del Tribunale di Firenze n. 6047 del 5/4/17 stampata da Tipografia Polistampa s.n.c. Firenze Direttore responsabile della rivista Consolata Siniscalco Comitato Editoriale Rubriche Responsabili Atti sociali Nicola Longo Attività societarie Segreteria della S.B.I. Biografie Giovanni Cristofolini Conservazione della Biodiversità vegetale Domenico Gargano, Gianni Bacchetta Didattica Silvia Mazzuca Disegno botanico Giovanni Cristofolini, Roberto Braglia Divulgazione e comunicazione di eventi, corsi, meeting futuri e relazioni Roberto Braglia Erbari Lorenzo Cecchi Giardini storici Paolo Grossoni Nuove Segnalazioni Floristiche Italiane Francesco RomaMarzio, Stefano Martellos Orti botanici Gianni Bedini Premi e riconoscimenti Segreteria della S.B.I. Recensioni di libri Paolo Grossoni Storia della Botanica Giovanni Cristofolini Tesi Botaniche Adriano Stinca Redazione Redattore Nicola Longo Coordinamento editoriale e impaginazione Monica Nencioni, Lisa Vannini, Chiara Barletta (Segreteria S.B.I.) Webmaster Roberto Braglia Sede via G. La Pira 4, 50121 Firenze Società Botanica Italiana onlus Via G. La Pira 4 – I 50121 Firenze – telefono 055 2757379 fax 055 2757378 email [email protected] – Home page http://www.societabotanicaitaliana.it Consiglio Direttivo Consolata Siniscalco (Presidente), Salvatore Cozzolino (Vice Presidente), Lorenzo -

Vascular Flora of Eight Water Reservoir Areas in Southern Italy
11 2 1593 the journal of biodiversity data February 2015 Check List LISTS OF SPECIES Check List 11(2): 1593, February 2015 doi: http://dx.doi.org/10.15560/11.2.1593 ISSN 1809-127X © 2015 Check List and Authors Vascular flora of eight water reservoir areas in southern Italy Antonio Croce Second University of Naples, Department of Environmental Biological and Pharmaceutical Sciences and Technologies, Via Vivaldi, 43, 8100 Caserta, Italy E-mail: [email protected] Abstract: Artificial lakes play an important role in Although many authors have reported the negative maintaining the valuable biodiversity linked to water impact of dams on rivers and their ecosystems (e.g., bodies and related habitats. The vascular plant diversity McAllister et al. 2001; Nilsson et al. 2005), dams are of eight reservoirs and surrounding areas in southern very important for wildlife, such as birds (Mancuso Italy was inventoried and further analysed in terms of 2010). Artificial lakes fulfill an important role as water biodiversity. A total of 730 specific and subspecific taxa reservoirs for agricultural irrigation; however, their were recorded, with 179 taxa in the poorest area and 303 other functions, such as recreation, fishing, and bio- in the richest one. The results indicate a good richness diversity conservation, should not be overlooked. The of the habitats surrounding the water basins, with some Italian National Institute for Economic Agriculture species of nature conservation interest and only a few (INEA) launched the project “Azione 7” (Romano and alien species. Costantini 2010) to assess the suitability of reservoirs in southern Italy for nature conservation purposes. -

Download from the ETH Zurich Research Collection
Research Collection Doctoral Thesis Tree regeneration on the flood plain of an alpine river Author(s): Karrenberg, Sophie Publication Date: 2002 Permanent Link: https://doi.org/10.3929/ethz-a-004339091 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14485 TREE REGENERATION ON THE FLOOD PLAINOF ANALPINERIVER A dissertation submitted to the Swiss Federal Institute of TechnologyZürich for the degree of Doctor of NaturalSciences presented by Sophie Karrenbergvan der Nat Diplom-Biologin, Christian-Albrechts-UniversitätKiel born August 5, 1972 in Germany accepted on the recommendation of Prof. P.J. Edwards,examiner Prof. J. Kollmann, co-examiner PD Dr. T. Speck, co-examiner 2002 Summary In many European rivers, regulation measures have led to the decline of braided channel Systems and associated pioneer ecosystems. An understanding of their natural functioning is necessary to develop appropriate management and restoration strategies. This thesis provides Informationon patterns of woody pioneer Vegetationand regeneration of the main pioneer trees of this habitat, i.e. the Grey Alder (Alnus incana, Betulaceae) and various species of the Salicaceae such as the Black Poplar (Populus nigra) and five species ofwillows (Salix). Within the active flood piain of a near-natural river (River Tagliamento, NE- Italy), we examined how woody Vegetation is impacted by environmental variables (Chapter 2). Alnus incana dominated the upper reaches of the river, whereas Populus nigra and Salix species were more abundant in the lower reaches. Woody Vegetationwas very young (mean <9 years) and mainly structuredby the longitudinal gradient, which was strongly correlated with mean annual temperature. -

The International Timber Trade
THE INTERNATIONAL TIMBER TRADE: A Working List of Commercial Timber Tree Species By Jennifer Mark1, Adrian C. Newton1, Sara Oldfield2 and Malin Rivers2 1 Faculty of Science & Technology, Bournemouth University 2 Botanic Gardens Conservation International The International Timber Trade: A working list of commercial timber tree species By Jennifer Mark, Adrian C. Newton, Sara Oldfield and Malin Rivers November 2014 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, TW9 3BW, UK Cover Image: Sapele sawn timber being put together at IFO in the Republic of Congo. Photo credit: Danzer Group. 1 Table of Contents Introduction ............................................................................................................ 3 Summary ................................................................................................................. 4 Purpose ................................................................................................................ 4 Aims ..................................................................................................................... 4 Considerations for using the Working List .......................................................... 5 Section Guide ...................................................................................................... 6 Section 1: Methods and Rationale .......................................................................... 7 Rationale - Which tree species are internationally traded for timber? ............. -

Willow Key) Website
Skvortsovia: 5(3): 71-82 (2020) Skvortsovia ISSN 2309-6497 (Print) Copyright: © 2020 Russian Academy of Sciences http://skvortsovia.uran.ru/ ISSN 2309-6500 (Online) Symposium proceedings The Lebbeke Salicetum and Wilgenzoeker (Willow Key) website Pol Meert Educational Salicetum (Educatief Wilgenarboretum), Lebbeke, Belgium Email: [email protected] Received: 6 March 2020 | Accepted by Irina Belyaeva: 14 September 2020 | Published online: 18 September 2020 Edited by: Irina Kadis, Irina Belyaeva and Keith Chamberlain Abstract A brief introduction to a project launched in 2009, The creation of a willow collection (Salicetum) in Lebbeke, Belgium and the website Wilgenzoeker (Willow Key) is given. Keywords: bees, education, Lebbeke, Salicaceae, Salicetum, Salix, website Wilgenzoeker Lebbeke Salicetum The creation and management of the Lebbeke Salicetum is a joint project between the local environmental organization, Natuurpunt, and the community of Lebbeke. The Salicetum is situated next to the Heizijde station on the Brussels-Dendermonde railway line. It lies along the Brabantsebeek, a creek of an above-ground water reservoir, one of six such reservoirs that were originally constructed to prevent flooding in the area. The erstwhile natural valley had disappeared following housing construction. To make the Salicetum more accessible, a walkway runs through it (Figs. 1–4). Figure 1. Walkway through the Salicetum, Figure 2. Entrance to the Salicetum, 17 May 2019 17 May 2019 71 Figure 3. View of the Salicetum, 17 May 2019 Figure 4. The Salicetum is situated along a railway line, 17 May 2019 The planting of Salix was begun in 2009 so that the plants would be in place for the International Year of Biodiversity which was to be celebrated throughout 2010 to draw attention to the importance of biological diversity.