Invasive Alien Species Clearing Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bruxner Park Flora Reserve Working Plan
Bruxner Park Flora Reserve Working Plan Working Plan for Bruxner Park Flora Reserve No 3 Upper North East Forest Agreement Region North East Region Contents Page 1. DETAILS OF THE RESERVE 2 1.1 Introduction 2 1.2 Location 2 1.3 Key Attributes of the Reserve 2 1.4 General Description 2 1.5 History 6 1.6 Current Usage 8 2. SYSTEM OF MANAGEMENT 9 2.1 Objectives of Management 9 2.2 Management Strategies 9 2.3 Management Responsibility 11 2.4 Monitoring, Reporting and Review 11 3. LIST OF APPENDICES 11 Appendix 1 Map 1 Locality Appendix 1 Map 2 Cadastral Boundaries, Forest Types and Streams Appendix 1 Map 3 Vegetation Growth Stages Appendix 1 Map 4 Existing Occupation Permits and Recreation Facilities Appendix 2 Flora Species known to occur in the Reserve Appendix 3 Fauna records within the Reserve Y:\Tourism and Partnerships\Recreation Areas\Orara East SF\Bruxner Flora Reserve\FlRWP_Bruxner.docx 1 Bruxner Park Flora Reserve Working Plan 1. Details of the Reserve 1.1 Introduction This plan has been prepared as a supplementary plan under the Nature Conservation Strategy of the Upper North East Ecologically Sustainable Forest Management (ESFM) Plan. It is prepared in accordance with the terms of section 25A (5) of the Forestry Act 1916 with the objective to provide for the future management of that part of Orara East State Forest No 536 set aside as Bruxner Park Flora Reserve No 3. The plan was approved by the Minister for Forests on 16.5.2011 and will be reviewed in 2021. -

Regional Landscape Surveillance for New Weed Threats Project 2016-2017
State Herbarium of South Australia Botanic Gardens and State Herbarium Economic & Sustainable Development Group Department of Environment, Water and Natural Resources Milestone Report Regional Landscape Surveillance for New Weed Threats Project 2016-2017 Milestone: Annual report on new plant naturalisations in South Australia Chris J. Brodie, Jürgen Kellermann, Peter J. Lang & Michelle Waycott June 2017 Contents Summary .................................................................................................................................... 3 1. Activities and outcomes for 2016/2017 financial year .......................................................... 3 Funding .................................................................................................................................. 3 Activities ................................................................................................................................ 4 Outcomes and progress of weeds monitoring ........................................................................ 6 2. New naturalised or questionably naturalised records of plants in South Australia. .............. 7 3. Description of newly recognised weeds in South Australia .................................................. 9 4. Updates to weed distributions in South Australia, weed status and name changes ............. 23 References ................................................................................................................................ 28 Appendix 1: Activities of the -
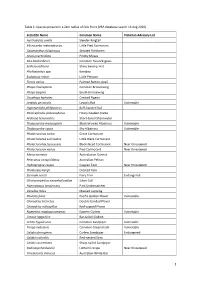
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Capitulo 3 Tesis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Digital.CSIC 1 Flowering phenology of invasive alien plant species compared to native 2 species in three mediterranean-type ecosystems 3 4 Oscar Godoy*1,4, David M. Richardson2, Fernando Valladares1,3 & Pilar Castro-Díez4 5 6 1 Laboratorio Internacional de Cambio Global (Linc-Global). Instituto de los Recursos 7 Naturales, Centro de Ciencias Medioambientales. CSIC. Serrano 115 dpdo E-28006 8 Madrid Spain. ! 9 2 Centre for Invasion Biology, Department of Botany & Zoology, Stellenbosch 10 University, Private Bag X1, Matieland 7602, South Africa. 11 3 Departamento de Biología y Geología. Área de Biodiversidad & Conservación, 12 Universidad Rey Juan Carlos, ESCET, Tulipán s/n E-28933, Móstoles, Madrid, Spain. 13 4 Departamento Interuniversitario de Ecología. Sección de Alcalá. Edificio de Ciencias. 14 Universidad de Alcalá, E-28871, Alcalá de Henares, Madrid, Spain. 15 16 *Correspondence author: [email protected] 17 18 19 20 21 22 23 24 25 26 27 28 29 1 1 Fenología de floración de las especies de plantas exóticas invasoras en 2 tres ecosistemas mediterráneos en comparación con las especies 3 nativas. 4 5 Resumen 6 • Antecedentes y Objetivos: La fenología de floración es un componente esencial 7 del éxito de las especies invasoras, ya que una elevada fecundidad incrementa su 8 potencial invasor. Por tanto, estudiamos la relación existente entre los patrones 9 de floración de las especies invasoras y nativas en tres regiones con clima 10 mediterráneo: California, España y la Región Sudafricana de El Cabo 11 • Métodos: 227 pares de especies invasoras-nativas fueron utilizados 12 • Resultados clave: Las especies invasoras tienen diferentes patrones de floración 13 en comparación con las especies nativas en las tres regiones. -

Henderson, L. (2007). Invasive, Naturalized and Casual Alien Plants in Southern Africa
Bothalia 37,2: 215–248 (2007) Invasive, naturalized and casual alien plants in southern Africa: a sum- mary based on the Southern African Plant Invaders Atlas (SAPIA) L. HENDERSON* Keywords: biomes, casual alien plants, invasive plants, Lesotho, naturalized plants, roadside surveys, SAPIA mapping project, South Africa, Swaziland ABSTRACT The primary objective of this publication is to provide an overview of the species identity, invasion status, geographical extent, and abundance of alien plants in South Africa, Swaziland and Lesotho, based on fi eld records from 1979 to the end of 2000. The dataset is all the species records for the study area in the Southern African Plant Invaders Atlas (SAPIA) database during this time period. A total of 548 naturalized and casual alien plant species were catalogued and invasion was recorded almost throughout the study area. Most invasion, in terms of both species numbers and total species abundance, was recorded along the southern, southwestern and eastern coastal belts and in the adjacent interior. This area includes the whole of the Fynbos and Forest Biomes, and the moister eastern parts of the Grassland and Savanna Biomes. This study reinforces previous studies that the Fynbos Biome is the most extensively invaded vegetation type in South Africa but it also shows that parts of Savanna and Grassland are as heavily invaded as parts of the Fynbos. The Fabaceae is prominent in all biomes and Acacia with 17 listed species, accounts for a very large proportion of all invasion. Acacia mearnsii was by far the most prominent invasive species in the study area, followed by A. -

Antarctic Beech (Nothofagus Moorei)
Dandarrga Nursery Native Species Labels A - M Antarctic Beech (Nothofagus moorei) Nothofagaceae A Gondwana rainforest tree 25 – 50 m Flowers Nov - Dec, seed pods Dec - Feb Range: High altitude rainforest of eastern Australia. This tree can reach a great age. New growth is red, and the complex root structure can grow multiple trunks, adorned with epiphytic orchids, ferns, fungi, mosses, liverworts and lichens. Bamboo Grass (Austrostipa ramosissima) Poaceae Native grass up to 1 to 2.5 m tall, 1.5 m wide Flowers: year round Range: S.E NSW to N.E QLD Stout Bamboo Grass is a tall ornamental grass. Fast growing and long lived. Useful container or border plant or for erosion and weed control. Attracts birds and small reptiles. Hardy; frost, drought and damp tolerant and grows in most soil conditions. Can be cut back hard to rejuvenate. Grows best with full or partial sun in shelter. Banana Bush (Tabernaemontana pandacaqui) Apocynaceae Deciduous shrub or small tree 1.5-14m Flowers: White; spring/summer Range: Manning River NSW to Cooktown QLD Normally growing to 1.5-3m in cultivation and can be pruned. Dense understory shrub with pretty tubular scented flowers. Unusual orange/ yellow fruit resemble small bananas but are poisonous to eat. Normally suitable for pruning. Adaptable to a range of moist, well-drained soil and prefers full or part shade. Dandarrga Nursery Native Species Labels A - M Basket Grass (Lomandra longifolia labill) Asparagaceae Native grass up to 1.2 m high & over 1m wide Flowers: cream to yellow from late winter to summer. Grows in a range of habitats FIRE RETARDANT SPECIES. -

Plants with Anti‑Leishmania Activity: Integrative Review from 2000 to 2011
PHCOG REV. REVIEW ARTICLE Plants with anti‑Leishmania activity: Integrative review from 2000 to 2011 Ana Maria G. Brito, Derivaldo dos Santos, Sheyla A. Rodrigues, Renan G. Brito1, Lauro Xavier-Filho Institute of Technology and Research, Department of Biomedicine, Tiradentes University, Aracaju-SE, 1 Department of Physiology, Federal University of Sergipe, São Cristóvão-SE, Brazil Submitted: 04-10-2012 Revised: 29-12-2012 Published: 01-06-2013 ABSTRACT The search for more effective new drugs to treat Leishmaniasis is undoubtedly relevant. Our objective in this study was to investigate research publications addressing plants with anti-Leishmaniasis activity. An integrative review of the literature from 2000 to 2011 was carried out in the databases such as Latin‑American and Caribbean Health Sciences (LILACS), Scientific Electronic Library Online (SciELO), and Medical Literature Analysis and Retrieval System Online (MEDLINE). In the initial search, 150 articles were found, with 25 based in LILACS, 68 in SciELO, and 46 in MEDLINE. From these data, after reading the abstracts that were available online, we excluded 12 from LILACS, 39 from SciELO, and 28 from MEDLINE for presenting article duplications. This left 61 articles to be read; however, only 18 of them answered the research questions and determined the final sample of this review. The results showed that research involving the search for new drugs against Leishmaniasis should be intensified, especially for the amastigote form, and studies with in vivo tests could become a great strategy for successfully finding new treatments for Leishmaniasis. It is believed that it is extremely important and urgent to conduct more trials in search of new effective drugs against Leishmaniasis that possess minimal adverse effects and that are easily accessible to the public. -

Cunninghamia Date of Publication: April 2020 a Journal of Plant Ecology for Eastern Australia
Cunninghamia Date of Publication: April 2020 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) A Systematic Flora Survey, Floristic Classification and High-Resolution Vegetation Map of Lord Howe Island Paul Sheringham 1*, Peter Richards2, Phil Gilmour3, Jill Smith1 and Ernst Kemmerer 4 1 Department of Planning, Industry and Environment, Locked Bag 914 COFFS HARBOUR NSW 2450 2 17 Coronation Avenue, SAWTELL NSW 2452 3 523 Roses Rd, GLENIFFER, NSW 2454 4 Cradle Coast NRM, PO Box 338, BURNIE TAS 7320 * Author for correspondence: [email protected] Abstract: The present study took advantage of the availability of high resolution ADS40 digital imagery to 1) systematically resample the vegetation of the Lord Howe Island Group (LHIG, excluding Ball’s Pyramid); 2) conduct a numerical analysis of the floristic data; 3) map vegetation extent and the distribution of vegetation communities and 4) compare the resultant classification and mapping with those of Pickard (1983). In July 2013, a total of 86 full floristic and 105 rapid floristic sites were sampled across the island, based on a stratified random sampling design. A hierarchical agglomerative clustering strategy (Flexible UPGMA) and Bray-Curtis dissimilarity coefficient with default beta, along with nearest neighbour analysis to identify anomalous site allocations, was used to analyze the floristic data. In total 33 vegetation communities were delineated and mapped: 19 mapping units from the full floristic analysis; 7 variants identified within five of the above 19 groups; 3 mapping units from analysis of canopy- only floristic data; and 4 mapping units recognised in previous studies that are mapped but were not sampled in this survey. -

Occasional Papers
NUMBER 79, 64 pages 27 July 2004 BISHOP MUSEUM OCCASIONAL PAPERS RECORDS OF THE HAWAII BIOLOGICAL SURVEY FOR 2003 PART 2: NOTES NEAL L. EVENHUIS AND LUCIUS G. ELDREDGE, EDITORS BISHOP MUSEUM PRESS HONOLULU C Printed on recycled paper Cover illustration: soldier of Coptotermes formosanus, the subterranean termite (modified from Williams, F.X., 1931, Handbook of the insects and other invertebrates of Hawaiian sugar cane fields). Bishop Museum Press has been publishing scholarly books on the natu- RESEARCH ral and cultural history of Hawai‘i and the Pacific since 1892. The Bernice P. Bishop Museum Bulletin series (ISSN 0005-9439) was begun PUBLICATIONS OF in 1922 as a series of monographs presenting the results of research in many scientific fields throughout the Pacific. In 1987, the Bulletin series BISHOP MUSEUM was superceded by the Museum’s five current monographic series, issued irregularly: Bishop Museum Bulletins in Anthropology (ISSN 0893-3111) Bishop Museum Bulletins in Botany (ISSN 0893-3138) Bishop Museum Bulletins in Entomology (ISSN 0893-3146) Bishop Museum Bulletins in Zoology (ISSN 0893-312X) Bishop Museum Bulletins in Cultural and Environmental Studies (NEW) (ISSN 1548-9620) Bishop Museum Press also publishes Bishop Museum Occasional Papers (ISSN 0893-1348), a series of short papers describing original research in the natural and cultural sciences. To subscribe to any of the above series, or to purchase individual publi- cations, please write to: Bishop Museum Press, 1525 Bernice Street, Honolulu, Hawai‘i 96817-2704, USA. Phone: (808) 848-4135. Email: [email protected]. Institutional libraries interested in exchang- ing publications may also contact the Bishop Museum Press for more information. -

Untersuchungen Zur Proteolytischen Aktivität Pflanzlicher Milchsäfte Und Deren Einfluss Auf Die Hämostase Und Fibrinolyse
Untersuchungen zur proteolytischen Aktivität pflanzlicher Milchsäfte und deren Einfluss auf die Hämostase und Fibrinolyse Dissertation zur Erlangung des akademischen Grades des Doktors der Naturwissenschaften (Dr. rer. nat.) eingereicht im Fachbereich Biologie, Chemie, Pharmazie der Freien Universität Berlin vorgelegt von Apotheker Martin Flemmig aus Mölln 2015 Die vorliegende Arbeit entstand in der Zeit von Oktober 2010 bis Mai 2015 unter der Leitung von Herrn Prof. Dr. Matthias F. Melzig am Institut für Pharmazie der Freien Universität Berlin. Erster Gutachter: Prof. Dr. Matthias F. Melzig Zweiter Gutachter: apl. Prof. Dr. Harshadrai M. Rawel Disputation am: 22.06.2015 Inhaltsverzeichnis Inhaltsverzeichnis Abkürzungsverzeichnis .......................................................................................................... IV 1. Einleitung ............................................................................................................................. 1 1.1 Milchsaft – Latex .......................................................................................................... 1 1.2 Euphorbiaceae JUSS. ...................................................................................................... 2 1.3 Proteasen ....................................................................................................................... 3 1.3.1 Einsatz von Proteasen als Therapeutika ............................................................. 3 1.3.2 Einsatz von Proteasen in anderen Bereichen ..................................................... -

Technical Report Series No. 287 Advisory List of Environmental Weeds in Victoria
Advisory list of environmental weeds in Victoria M. White, D. Cheal, G.W. Carr, R. Adair, K. Blood and D. Meagher April 2018 Arthur Rylah Institute for Environmental Research Technical Report Series No. 287 Arthur Rylah Institute for Environmental Research Department of Environment, Land, Water and Planning PO Box 137 Heidelberg, Victoria 3084 Phone (03) 9450 8600 Website: www.ari.vic.gov.au Citation: White, M., Cheal, D., Carr, G. W., Adair, R., Blood, K. and Meagher, D. (2018). Advisory list of environmental weeds in Victoria. Arthur Rylah Institute for Environmental Research Technical Report Series No. 287. Department of Environment, Land, Water and Planning, Heidelberg, Victoria. Front cover photo: Ixia species such as I. maculata (Yellow Ixia) have escaped from gardens and are spreading in natural areas. (Photo: Kate Blood) © The State of Victoria Department of Environment, Land, Water and Planning 2018 This work is licensed under a Creative Commons Attribution 3.0 Australia licence. You are free to re-use the work under that licence, on the condition that you credit the State of Victoria as author. The licence does not apply to any images, photographs or branding, including the Victorian Coat of Arms, the Victorian Government logo, the Department of Environment, Land, Water and Planning logo and the Arthur Rylah Institute logo. To view a copy of this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Printed by Melbourne Polytechnic, Preston Victoria ISSN 1835-3827 (print) ISSN 1835-3835 (pdf)) ISBN 978-1-76077-000-6 (print) ISBN 978-1-76077-001-3 (pdf/online) Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication.