Neuronal Cell-Type Classification: Challenges, Opportunities and the Path Forward
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Building Atlases of the Brain
© Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. BUILDING AtLASES OF THE BRAIN Mike Hawrylycz With Chinh Dang, Christof Koch, and Hongkui Zeng A Very Brief History of Brain Atlases The earliest known significant works on human anatomy were collected by the Greek physician Claudius Galen around 200 BCE. This ancient corpus remained the dominant viewpoint through the Middle Ages until the classic work De humani corporis fabrica (On the Fabric of the Human Body) by Andreas Vesalius of Padua (1514–1564), the first mod- ern anatomist. Even today many of Vesalius’s drawings are astonishing to study and are largely accurate. For nearly two centuries scholars have recognized that the brain is compartmentalized into distinct regions, and this organization is preserved throughout mammals in general. However, comprehending the structural organization and function of the nervous system remains one of the primary challenges in neuro- science. To analyze and record their findings neuroanatomists develop atlases or maps of the brain similar to those cartographers produce. The state of our understanding today of an integrated plan of brain function remains incomplete. Rather than indicating a lack of effort, this observation highlights the profound complexity and interconnec- tivity of all but the simplest neural structures. Laying the foundation of cellular neuroscience, Santiago Ramón y Cajal (1852– 1934) drew and classified many types of neurons and speculated that the brain consists of an interconnected network of distinct neurons, as opposed to a more continuous web. -

2007 Lake Monitoring Report Mecklenburg County Water Quality Program SWIM Phase I Part 2-CO
2007 Lake Monitoring Report Mecklenburg County Water Quality Program SWIM Phase I Part 2-CO Prepared by: David Buetow Final Report for FY2007-2008 PURPOSE AND BACKGROUND The Mecklenburg County Water Quality Program (MCWQP) has an ongoing program to monitor the water quality in the three Catawba Lakes bordering the county: Lake Norman, Mountain Island Lake and Lake Wylie. Samples are also routinely collected at the two water bodies in the northern end of Mecklenburg County cut off from Lake Norman proper by I-77: Lake Cornelius and Lake Davidson. Data collected from all lake sites are used to screen for environmental problems using MCWQP Action and Watch levels for various pollutants and also to rate the overall water quality at sampling locations in the lakes using a water quality index. Additional objectives are to describe seasonal trends and address spatial variability in the data. This report presents the lake monitoring data for the calendar year 2007 ACTIVITIES AND METHODS Regular monthly lake sampling was conducted at seven locations in Lake Norman and one location each on Lake Cornelius and Lake Davidson (Appendix A), ten locations in Mountain Island Lake (Appendix B) and eight sampling locations in Lake Wylie (Appendix C). In May 2007, the sampling program was changed in Lake Wylie to add several new cove monitoring sites. This resulted in an increase in regular sampling sites in Lake Wylie from eight to thirteen (Appendix D). Lakes Norman, Wylie and Mountain Island were sampled monthly from May through September and every other month during the rest of the year, i.e. -

Cortical Bitufted, Horizontal, and Martinotti Cells Preferentially
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3217–3222, March 1999 Neurobiology Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete reelin into perineuronal nets, nonsynaptically modulating gene expression (GABAergic interneuronsyCa21-binding proteinsyneuropeptidesychandelier cellsybasket cells) CHRISTINE PESOLD*†,WEN SHENG LIU*, ALESSANDRO GUIDOTTI*, ERMINIO COSTA*, AND HECTOR J. CARUNCHO*‡ *Psychiatric Institute, Department of Psychiatry, College of Medicine, University of Illinois at Chicago, 1601 West Taylor Street, MyC 912, Chicago IL, 60612; and ‡Department of Fundamental Biology, University of Santiago de Compostela, Galicia, Spain 15706 Contributed by Erminio Costa, December 28, 1998 ABSTRACT Reelin (Reln) is a protein with some struc- mouse, shows a normal Reln expression in Cajal–Retzius cells tural analogies with other extracellular matrix proteins that and secretion in the extracellular matrix in embryonic devel- functions in the regulation of neuronal migration during the opment but neuroanatomical abnormalities reminiscent of development of cortical laminated structures. In the cortex of those in the reeler mouse (4, 5). It is currently believed that the adult animals, Reln is expressed primarily in g-aminobutyric protein encoded by this gene acts downstream of Reln in a acid (GABA)ergic neurons and is secreted into perineuronal signaling pathway that controls laminar corticogenesis and the nets. However, only 50–60% of GABAergic interneurons ex- hippocampal and cerebellar development in mammalian brain press Reln. We have characterized this subpopulation of (6). cortical GABAergic neurons that expresses Reln by using two In adult telencephalon, Reln is preferentially expressed in strategies: (i) a double immunolabeling procedure to deter- GABAergic neurons (7, 8), and Dab1 is expressed predomi- mine the colocalization of Reln with neuropeptides and Ca21- nantly in pyramidal cells, as well as in a small population of binding proteins and (ii) a combination of Golgi staining and non-pyramidal cells (9). -

Introduction to the Cell Cell History Cell Structures and Functions
Introduction to the cell cell history cell structures and functions CK-12 Foundation December 16, 2009 CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and distribution of high quality educational content that will serve both as core text as well as provide an adaptive environment for learning. Copyright ©2009 CK-12 Foundation This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/by-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA. Contents 1 Cell structure and function dec 16 5 1.1 Lesson 3.1: Introduction to Cells .................................. 5 3 www.ck12.org www.ck12.org 4 Chapter 1 Cell structure and function dec 16 1.1 Lesson 3.1: Introduction to Cells Lesson Objectives • Identify the scientists that first observed cells. • Outline the importance of microscopes in the discovery of cells. • Summarize what the cell theory proposes. • Identify the limitations on cell size. • Identify the four parts common to all cells. • Compare prokaryotic and eukaryotic cells. Introduction Knowing the make up of cells and how cells work is necessary to all of the biological sciences. Learning about the similarities and differences between cell types is particularly important to the fields of cell biology and molecular biology. -

Introduction to Class S Law
Introduction to Class S Law 1 This schedule is the result of a rigorous and detailed analysis of the the vocabulary of law, using the techniques of facet analysis. As such, it represents a radical revision and expansion of Class S in the first edition of the Bibliographic Classification (BC1)1. The general reasons for making the revision so radical a one are given in the Introduction and Auxiliary schedules2. The particular changes in this class are considered in Section 15 below. 2 The Outline on page 1 (after the preliminary pages) is designed to give a clear view of the basic structure. If it is remembered that the schedule is an inverted one (see Section 8) the outline will be seen to show not only the general sequence of facets (categories) and their classes but also the basic operational rule in applying the classification. This is the rule that compound classes (those reflecting the intersection of two or more simpler classes) are located under the class appearing later (lower down) in the schedule. For example, Contract law is SBE; English law is SN; so the compound class English law of contract goes under English law (at SNE) and not under Contract. Similarly, Evidence is S8T; Criminal law is SBW; so the compound class Evidence in criminal law goes under Criminal law (at SBW 8T) and not under Evidence. 3 Scope of Class S and its place in BC2 3.1 The law considered in Class S may be defined briefly as the enforceable body of rules that governs any society. -
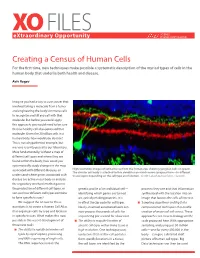
Creating a Census of Human Cells
XO FILES SCIENCE eXtraordinary Opportunity PHILANTHROPY ALLIANCE Creating a Census of Human Cells For the first time, new techniques make possible a systematic description of the myriad types of cells in the human body that underlie both health and disease. Aviv Regev Imagine you had a way to cure cancer that involved taking a molecule from a tumor and engineering the body’s immune cells to recognize and kill any cell with that molecule. But before you could apply this approach, you would need to be sure that no healthy cell also expressed that molecule. Given the 20 trillion cells in a human body, how would you do that? This is not a hypothetical example, but was one recently posed to our laboratory. More fundamentally, without a map of different cell types and where they are found within the body, how could you systematically study changes in the map associated with different diseases, or High resolution images of retinal tissue from the human eye, showing ganglion cells (in green). The circular cell body is attached to thin dendrites on which nerve synapses form—in different understand where genes associated with tissue layers depending on the cell type and function. Credit: Lab of Joshua Sanes, Harvard. disease are active in our body or analyze the regulatory mechanisms that govern the production of different cell types, or genetic profile of an individual cell— proteins they use and that information sort out how different cell types combine identifying which genes are turned synthesized with the location into an to form specific tissues? on, actively making proteins. -

Epidermal Growth Factor Promotes a Neural Phenotype in Thymic
Epidermal Growth Factor Promotes a Neural Phenotype in Thymic Epithelial Cells and Enhances Neuropoietic Cytoldne Expression Isabella Screpanti,* Susanna Scarpa,* Daniela Meco,* Diana BeUavia,~ Liborio Stuppia, § Luigi Frati, *u Andrea Modesti,* and Alberto Gulino I *Department of Experimental Medicine and Pathology, University La Sapienza, 00161 Rome;qnstitute of Human Pathology and Social Medicine, University of Chieti, 66100 Chieti; §Institute of N.P. Human Cytomorphology, National Research Council, 66100 Chieti; and UMediterranean Institute of Neuroscience, Pozzilli and IDepartment of Experimental Medicine, University of L'Aquila, 67100 L'Aquila, Italy Abstract. Neural crest-derived cells populate the thy- growth factor enhances cells that express the genes en- Downloaded from http://rupress.org/jcb/article-pdf/130/1/183/1264385/183.pdf by guest on 29 September 2021 mus, and their coexistence with epithelial cells is re- coding the preprotachykinin A-generated neuropep- quired for proper organ development and T cell educa- tides and the bipotential neuropoietic and lymphopoi- tion function. We show here that epidermal growth etic cytokines ciliary neurotrophic factor and factor (EGF), a major epithelial cell growth-enhancing interleukin-6. These cytokines also enhance the neu- agent, has a morphogenetic action to promote the ex- ronal phenotype of thymic epithelial cells. Therefore, pression of a neuronal phenotype (e.g., neurofilament EGF appears to be a composite autocrine/paracrine expression) in cultured thymic epithelial cells that are neuromodulator in thymic stroma. This suggests that characterized by a cytokeratin-positive epithelial cell EGF may regulate thymus-dependent immune func- background. The proliferation of such neurodifferenti- tions by promoting neuronal gene expression in neural ated cells is also enhanced by EGF. -

Classification Made Easy Class 1
Classification Made Easy Class 1 (CP1) The most severely disabled athletes belong to this classification. These athletes are dependent on a power wheelchair or assistance for mobility. They have severe limitation in both the arms and the legs and have very poor trunk control. Sports Available: • Race Runner (RR1) – using the Race Runner frame to run, track events include 100m, 200m and 400m. • Boccia o Boccia Class 1 (BC1) – players who fit into this category can throw the ball onto the court or a CP2 Lower who chooses to push the ball with the foot. Each BC1 athlete has a sport assistant on court with them. o Boccia Class 3 (BC3) – players who fit into this category cannot throw the ball onto the court and have no sustained grasp or release action. They will use a “chute” or “ramp” with the help from their sport assistant to propel the ball. They may use head or arm pointers to hold and release the ball. Players with a impairment of a non cerebral origin, severely affecting all four limbs, are included in this class. Class 2 (CP2) These athletes have poor strength or control all limbs but are able to propel a wheelchair. Some Class 2 athletes can walk but can never run functionally. The class 2 athletes can throw a ball but demonstrates poor grasp and release. Sports Available: • Race Runner (RR2) - using the Race Runner frame to run, track events include 100m, 200m and 400m. • Boccia o Boccia Class 2 (BC2) – players can throw the ball into the court consistently and do not need on court assistance. -

Stem Cells and Neurological Disease the Transplant Site
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.5.557 on 1 May 2003. Downloaded from EDITORIAL 553 Stem cells shown to survive and ameliorate behav- ................................................................................... ioural deficits in an animal mode of Par- kinson’s disease,3 although in this study 20% of rats still developed teratomas at Stem cells and neurological disease the transplant site. In contrast, Kim et al, using a different approach that relies on R A Barker, M Jain,RJEArmstrong, M A Caldwell transfection with Nurr1 (a transcription ................................................................................... factor involved in the differentiation of dopaminergic cells), have demonstrated The therapeutic implications and application of stem cells for functional efficacy without tumour formation.4 the nervous system Human embryonic stem cells have now been isolated5 and grown in culture with enrichment for neuronal lineages, here has recently been a great deal of (c) ability to migrate and disseminate possible through exposure to a combina- interest in stem cells and the nerv- following implantation within the adult tion of growth factors and mitogens.6 Tous system, in terms of their poten- CNS; These cells, when placed in the develop- tial for deciphering developmental issues (d) possible tropism for areas of path- ing rat brain, can migrate widely and as well as their therapeutic potential. In ology; differentiate in a site specific fashion this editorial we will critically appraise without the formation of teratomas.7 the different types of stem cells, their (e) ease of manipulation using viral and non-viral gene transfer methods; However, the safety of these cells needs therapeutic implications, and the appli- further investigation before they can be (f) ability to better integrate into normal cations to which they have been put, considered for clinical use. -

Robustness and Timing of Cellular Differentiation Through Population-Based Symmetry Breaking
AR TI CLE Robustness AND TIMING OF CELLULAR DIFFERENTIATION THROUGH population-based SYMMETRY BREAKING Angel StanoeV1 | Christian Schröter1 | Aneta KOSESKA1∗ 1Department OF Systemic Cell Biology, Max Planck Institute OF Molecular PhYSIOLOGY, Although COORDINATED BEHAVIOR OF MULTICELLULAR ORGANISMS Dortmund, GermanY RELIES ON INTERCELLULAR communication, THE DYNAMICAL mech- Correspondence ANISM THAT UNDERLIES SYMMETRY BREAKING IN HOMOGENEOUS Email: populations, THEREBY GIVING RISE TO HETEROGENEOUS CELL TYPES ∗[email protected] REMAINS UNCLEAR. In CONTRAST TO THE PREVALENT cell-intrinsic VIEW OF DIFFERENTIATION WHERE MULTISTABILITY ON THE SINGLE CELL LEVEL IS A NECESSARY pre-requisite, WE PROPOSE THAT het- EROGENEOUS CELLULAR IDENTITIES EMERGE AND ARE MAINTAINED COOPERATIVELY ON A POPULATION LEVel. Identifying A GENERIC SYMMETRY BREAKING mechanism, WE DEMONSTRATE THAT ROBUST PROPORTIONS BETWEEN DIFFERENTIATED CELL TYPES ARE INTRINSIC FEATURES OF THIS INHOMOGENEOUS STEADY STATE solution. Crit- ICAL ORGANIZATION IN THE VICINITY OF THE SYMMETRY BREAKING BIFURCATION ON THE OTHER hand, SUGGESTS THAT TIMING OF cel- LULAR DIFFERENTIATION EMERGES FOR GROWING POPULATIONS IN A self-organized MANNER. Setting A CLEAR DISTINCTION BETWEEN pre-eXISTING ASYMMETRIES AND SYMMETRY BREAKING EVents, WE THUS DEMONSTRATE THAT EMERGENT SYMMETRY breaking, IN CONJUNCTION WITH ORGANIZATION NEAR CRITICALITY NECESSARILY LEADS TO ROBUSTNESS AND ACCURACY DURING DEVelopment. KEYWORDS SYMMETRY breaking; differentiation; INHOMOGENEOUS STEADY STATE Abbreviations: -

A Model of Long-Term Memory Storage in the Cerebellar Cortex: a Possible Role for Plasticity at Parallel Fiber Synapses Onto Stellate͞basket Interneurons
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14200–14205, December 1997 Psychology A model of long-term memory storage in the cerebellar cortex: A possible role for plasticity at parallel fiber synapses onto stellateybasket interneurons GARRETT T. KENYON† Department of Neurobiology and Anatomy, University of Texas Medical School at Houston, 6431 Fannin, Houston, TX 77030 Edited by Richard F. Thompson, University of Southern California, Los Angeles, CA, and approved October 3, 1997 (received for review April 14, 1997) ABSTRACT By evoking changes in climbing fiber activity, Despite evidence supporting their postulated role in motor movement errors are thought to modify synapses from parallel learning, theoretical arguments suggest that memories stored fibers onto Purkinje cells (pf*Pkj) so as to improve subse- at pf*Pkj synapses would be susceptible to long-term degra- quent motor performance. Theoretical arguments suggest dation as a result of ongoing motor adaptation. First, motor there is an intrinsic tradeoff, however, between motor adap- memories are likely to be distributed across overlapping sets of tation and long-term storage. Assuming a baseline rate of pf*Pkj synapses. If motor memories did not overlap, the motor errors is always present, then repeated performance of storage capacity of pf*Pkj synapses would be greatly reduced any learned movement will generate a series of climbing (12, 17). Second, it is reasonable to assume that the pattern of fiber-mediated corrections. By reshuffling the synaptic synaptic weights necessary to effect any given movement is weights responsible for any given movement, such corrections non-unique. If the activity of a Purkinje cell depends only on will degrade the memories for other learned movements stored the total sum of the synaptic input at any given moment, then in overlapping sets of synapses. -

Complete Dissertation
VU Research Portal Cholinergic modulation of microcircuits in the cortex Obermayer, J.M.G. 2019 document version Publisher's PDF, also known as Version of record Link to publication in VU Research Portal citation for published version (APA) Obermayer, J. M. G. (2019). Cholinergic modulation of microcircuits in the cortex. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. E-mail address: [email protected] Download date: 04. Oct. 2021 Cholinergic modulation of microcircuits in the cortex Joshua Obermayer The research described in this thesis was conducted at the department of Integrative Neurophysiology of the Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, Vrije Universiteit Amsterdam, the Netherlands. No part of this thesis may be reproduced without prior permission of the author. Front cover: shows two pyramidal neurons and an interneuron that together form a disynaptic inhibitory microcircuit in the human cortex.