How Neutrophils Meet Their End
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Victor Nizet, M.D
Victor Nizet, M.D. Professor & Vice Chair for Basic Research, Department of Pediatrics Chief, Division of Host-Microbe Systems & Therapeutics University of California, San Diego School of Medicine Professor, Skaggs School of Pharmacy & Pharmaceutical Sciences Biomedical Research Facility II Building, Room 4113 Mail Code 0760, 9500 Gilman Drive, La Jolla, CA 92093-0760 Phone: (858) 534-7408; FAX: (858) 246-1868; email: [email protected] URL: http://nizetlab.ucsd.edu PERSONAL Born Dec 24, 1962 in Augusta, Georgia, USA Married (Christine), Son (Oliver) MAJOR AREAS OF ACADEMIC INTEREST The interest of my laboratory lies in understanding fundamental mechanisms of bacterial pathogenesis and the innate immune system, with a special focus on human streptococcal and staphylococcal infections and emerging antibiotic-resistant pathogens. Using a wide variety of molecular genetic approaches, the laboratory discovers and characterizes bacterial virulence determinants involved in cytotoxicity, adherence, invasion, inflammation, molecular mimicry and resistance to immunologic clearance. In companion studies, we investigate the contribution of host factors such as antimicrobial peptides, cytokines, leukocyte surface receptors, signal transduction pathways, and transcription factors in defense against invasive bacterial infection. Ultimately, we believe the basic discovoeries gained through this platform will inform novel treatment strategies for infectious diseases, including targeted neutralization of microbial virulence phenotypes, pharmacologic augmentation of host immune function, novel monoclonal antibodies and vaccines, and repurposing of FDA-approved drugs to beneficial impact at the host-pathogen interface. My other longstanding academic focus areas include cross-disciplinary research and educational program development, enhancing graduate and postdoctoral training in biological, medical and pharmaceutical sciences, junior faculty development, encouragement of academic-industry collaborations, and public engagement in the sciences. -

Innate Antimicrobial Peptide Protects the Skin from Invasive Bacterial Infection
letters to nature ................................................................. injections of cathelicidin-sensitive GAS in wild-type, heterozygous Innate antimicrobial peptide and homozygous-null mice, CRAMP-de®cient mice were observed to develop much larger areas of infection (Fig. 2a, b). Lesion areas protects the skin from increased more rapidly, reached larger maximal size, and persisted longer in CRAMP-de®cient mice than in normal littermates while invasive bacterial infection heterozygotes tended to have lesions of intermediate size (Fig. 2c). Cultures of equal amounts of tissue from lesions biopsied at day 7 Victor Nizet*, Takaaki Ohtake²³, Xavier Lauth²³, Janet Trowbridge²³, after injection demonstrated persistent infection with b-haemolytic Jennifer Rudisill²³, Robert A. Dorschner²³, Vasumati Pestonjamasp²³, GAS in CRAMP-de®cient mice but not in normal mice (Fig. 2d). No Joseph Piraino§, Kenneth Huttner§ & Richard L. Gallo*²³ difference in GAS lesion size was seen when wild-type parental strains C57BL/6 and 129/SVJ were compared. * Department of Pediatrics; and ² Division of Dermatology, A complementary approach to demonstrating the importance of University of California, San Diego, California 92161, USA cathelicidins in host defence is to examine the effects in vivo of ³ Veterans Affairs San Diego Healthcare System, San Diego, California 92161, altering bacterial sensitivity to CRAMP. If the antimicrobial action USA of cathelicidin is essential to control a GAS skin infection, as § Division of Neonatology, Massachusetts -

Endogenous Production of Antimicrobial Peptides in Innate Immunity and Human Disease Richard L
Endogenous Production of Antimicrobial Peptides in Innate Immunity and Human Disease Richard L. Gallo, MD, PhD, and Victor Nizet, MD Address The definition of an AMP has been loosely applied to Departments of Medicine and Pediatrics, Division of Dermatology, Uni- any peptide with the capacity to inhibit the growth of versity of California San Diego, and VA San Diego Healthcare System, microbes. AMPs might exhibit potent killing or inhibi- San Diego, CA, USA. E-mail: [email protected] tion of a broad range of microorganisms, including gram- positive and -negative bacteria as well as fungi and certain Current Allergy and Asthma Reports 2003, 3:402–409 Current Science Inc. ISSN 1529-7322 viruses. More than 800 such AMPs have been described, Copyright © 2003 by Current Science Inc. and an updated list can be found at the website: http:// www.bbcm.units.it/~tossi/pag1.htm. Some of these AMPs Antimicrobial peptides are diverse and evolutionarily ancient have now been demonstrated to protect diverse organ- molecules produced by all living organisms. Peptides belong- isms, including plants, insects, and mammals, against ing to the cathelicidin and defensin gene families exhibit an infection. AMP sequence analysis has shown that the pep- immune strategy as they defend against infection by inhibiting tide immune system is evolutionarily ancient, and the microbial survival, and modify hosts through triggering tis- conservation of AMP gene families throughout the ani- sue-specific defense and repair events. A variety of processes mal kingdom further supports their biologic significance. have evolved in microbes to evade the action of antimicrobial Antimicrobial peptides function within the conceptual peptides, including the ability to degrade or inactivate antimi- framework of the innate immune system. -

3970.Full.Pdf
Cellular Activation, Phagocytosis, and Bactericidal Activity Against Group B Streptococcus Involve Parallel Myeloid Differentiation Factor 88-Dependent and This information is current as Independent Signaling Pathways of September 27, 2021. Philipp Henneke, Osamu Takeuchi, Richard Malley, Egil Lien, Robin R. Ingalls, Mason W. Freeman, Tanya Mayadas, Victor Nizet, Shizuo Akira, Dennis L. Kasper and Douglas T. Golenbock Downloaded from J Immunol 2002; 169:3970-3977; ; doi: 10.4049/jimmunol.169.7.3970 http://www.jimmunol.org/content/169/7/3970 http://www.jimmunol.org/ References This article cites 61 articles, 36 of which you can access for free at: http://www.jimmunol.org/content/169/7/3970.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 27, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2002 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Cellular Activation, Phagocytosis, and Bactericidal Activity Against Group B Streptococcus Involve Parallel Myeloid Differentiation Factor 88-Dependent and Independent Signaling Pathways1 Philipp Henneke,*† Osamu Takeuchi,‡ Richard Malley,§ Egil Lien,* Robin R. -

Antimicrobial Defense by Phagocyte Extracellular Traps
J Mol Med DOI 10.1007/s00109-009-0481-0 REVIEW Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps Maren von Köckritz-Blickwede & Victor Nizet Received: 11 March 2009 /Revised: 15 April 2009 /Accepted: 23 April 2009 # The Author(s) 2009. This article is published with open access at Springerlink.com Abstract The formation of extracellular traps (ETs) by Keywords Neutrophil . Mast cell . Eosinophil . phagocytic cells has been recognized as a novel and Extracellular trap . Innate immunity. Bacterial infection . important mechanism of the host innate immune response Virulence factors . Antimicrobial peptides . DNA . Histones . against infections. ETs are formed by different host immune Mitochondria cells such as neutrophils, mast cells, and eosinophils after stimulation with mitogens, cytokines, or pathogens them- selves, in a process dependent upon induction of a reactive- Introduction oxygen-species-mediated signaling cascade. ETs consist of nuclear or mitochondrial DNA as a backbone with The frontline function of phagocytes such as neutrophils embedded antimicrobial peptides, histones, and cell- and macrophages in our innate immune defense has been specific proteases and thereby provide a matrix to entrap classically understood to reflect a variety of potent and kill microbes and to induce the contact system. This intracellular microbicidal mechanisms. Upon contact with review summarizes the latest research on ETs and their role the invading pathogen, phagocytes engulf the microbes into in innate immunity and host innate defense. Attention is their phagocytic vacuoles (phagosomes). Efficient uptake is also given to mechanisms by which certain leading facilitated through prior opsonization of the microbe with bacterial pathogens have evolved to avoid entrapment and circulating complement or, in the nonnaïve host, specific killing in these specialized structures. -

The Impact of Hypoxia on Neutrophil Degranulation and Consequences for the Host
International Journal of Molecular Sciences Review The Impact of Hypoxia on Neutrophil Degranulation and Consequences for the Host Katharine M. Lodge 1, Andrew S. Cowburn 1 , Wei Li 2 and Alison M. Condliffe 3,* 1 Faculty of Medicine, National Heart and Lung Institute, Imperial College London, London SW3 6LY, UK; [email protected] (K.M.L.); [email protected] (A.S.C.) 2 Department of Medicine, University of Cambridge, Cambridge CB2 0SP, UK; [email protected] 3 Department of Infection, Immunity and Cardiovascular Diseases, University of Sheffield, Sheffield S10 2RX, UK * Correspondence: a.m.condliffe@sheffield.ac.uk Received: 13 January 2020; Accepted: 8 February 2020; Published: 11 February 2020 Abstract: Neutrophils are key effector cells of innate immunity, rapidly recruited to defend the host against invading pathogens. Neutrophils may kill pathogens intracellularly, following phagocytosis, or extracellularly, by degranulation and the release of neutrophil extracellular traps; all of these microbicidal strategies require the deployment of cytotoxic proteins and proteases, packaged during neutrophil development within cytoplasmic granules. Neutrophils operate in infected and inflamed tissues, which can be profoundly hypoxic. Neutrophilic infiltration of hypoxic tissues characterises a myriad of acute and chronic infectious and inflammatory diseases, and as well as potentially protecting the host from pathogens, neutrophil granule products have been implicated in causing collateral tissue damage in these scenarios. This review discusses the evidence for the enhanced secretion of destructive neutrophil granule contents observed in hypoxic environments and the potential mechanisms for this heightened granule exocytosis, highlighting implications for the host. Understanding the dichotomy of the beneficial and detrimental consequences of neutrophil degranulation in hypoxic environments is crucial to inform potential neutrophil-directed therapeutics in order to limit persistent, excessive, or inappropriate inflammation. -
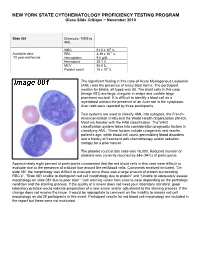
10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

Characterization of Early-Phase Neutrophil Extracellular Traps in Urinary Tract Infections
RESEARCH ARTICLE Characterization of Early-Phase Neutrophil Extracellular Traps in Urinary Tract Infections Yanbao Yu, Keehwan Kwon, Tamara Tsitrin, Shiferaw Bekele, Patricia Sikorski¤, Karen E. Nelson, Rembert Pieper* The J. Craig Venter Institute, Rockville, MD, United States of America ¤ Current address: Laboratory of Parasitic Diseases, NIAID, NIH, Bethesda, MD; Department of Microbiology and Immunology, Georgetown University, N.W., Washington, DC * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 Neutrophils have an important role in the antimicrobial defense and resolution of urinary tract infections (UTIs). Our research suggests that a mechanism known as neutrophil extra- cellular trap (NET) formation is a defense strategy to combat pathogens that have invaded the urinary tract. A set of human urine specimens with very high neutrophil counts had OPEN ACCESS microscopic evidence of cellular aggregation and lysis. Deoxyribonuclease I (DNase) treat- Citation: Yu Y, Kwon K, Tsitrin T, Bekele S, Sikorski ment resulted in disaggregation of such structures, release of DNA fragments and a prote- P, Nelson KE, et al. (2017) Characterization of ome enriched in histones and azurophilic granule effectors whose quantitative composition Early-Phase Neutrophil Extracellular Traps in Urinary Tract Infections. PLoS Pathog 13(1): was similar to that of previously described in vitro-formed NETs. The effector proteins were e1006151. doi:10.1371/journal.ppat.1006151 further enriched in DNA-protein complexes isolated in native PAGE gels. Immunofluores- Editor: David Weiss, Emory University School of cence microscopy revealed a flattened morphology of neutrophils associated with decon- Medicine, UNITED STATES densed chromatin, remnants of granules in the cell periphery, and myeloperoxidase co- Received: April 5, 2016 localized with extracellular DNA, features consistent with early-phase NETs. -

Neutrophil Azurophilic Granule Glycoproteins Are Distinctively Decorated by Atypical Pauci- and Phosphomannose Glycans ✉ Karli R
ARTICLE https://doi.org/10.1038/s42003-021-02555-7 OPEN Neutrophil azurophilic granule glycoproteins are distinctively decorated by atypical pauci- and phosphomannose glycans ✉ Karli R. Reiding 1,2,4 , Yu-Hsien Lin1,2,4, Floris P. J. van Alphen3, Alexander B. Meijer1,3 & ✉ Albert J. R. Heck 1,2 While neutrophils are critical first-responders of the immune system, they also cause tissue damage and act in a variety of autoimmune diseases. Many neutrophil proteins are N-glycosylated, a post-translational modification that may affect, among others, enzymatic 1234567890():,; activity, receptor interaction, and protein backbone accessibility. So far, a handful neutrophil proteins were reported to be decorated with atypical small glycans (paucimannose and smaller) and phosphomannosylated glycans. To elucidate the occurrence of these atypical glycoforms across the neutrophil proteome, we performed LC-MS/MS-based (glyco)proteomics of pooled neutrophils from healthy donors, obtaining site-specific N-glycan characterisation of >200 glycoproteins. We found that glycoproteins that are typically membrane-bound to be mostly decorated with high-mannose/complex N-glycans, while secreted proteins mainly harboured complex N-glycans. In contrast, proteins inferred to originate from azurophilic granules carried distinct and abundant paucimannosylation, asymmetric/hybrid glycans, and glycan phospho- mannosylation. As these same proteins are often autoantigenic, uncovering their atypical gly- cosylation characteristics is an important step towards understanding autoimmune disease and improving treatment. 1 Biomolecular Mass Spectrometry and Proteomics, Bijvoet Center for Biomolecular Research and Utrecht Institute for Pharmaceutical Sciences, University of Utrecht, Utrecht, The Netherlands. 2 Netherlands Proteomics Center, Utrecht, The Netherlands. 3 Department of Molecular and Cellular Hemostasis, Sanquin ✉ Research, Amsterdam, The Netherlands. -

Host Antimicrobial Peptides: the Promise of New Treatment Strategies Against Tuberculosis
REVIEW published: 07 November 2017 doi: 10.3389/fimmu.2017.01499 Host Antimicrobial Peptides: The Promise of New Treatment Strategies against Tuberculosis Javier Arranz-Trullén1,2‡, Lu Lu1‡, David Pulido1†, Sanjib Bhakta 2* and Ester Boix 1* 1 Faculty of Biosciences, Department of Biochemistry and Molecular Biology, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, Spain, 2 Mycobacteria Research Laboratory, Department of Biological Sciences, Institute of Structural and Molecular Biology, Birkbeck University of London, London, United Kingdom Edited by: Tuberculosis (TB) continues to be a devastating infectious disease and remerges as a Uday Kishore, global health emergency due to an alarming rise of antimicrobial resistance to its treat- Brunel University London, ment. Despite of the serious effort that has been applied to develop effective antitubercular United Kingdom chemotherapies, the potential of antimicrobial peptides (AMPs) remains underexploited. Reviewed by: Suraj Sable, A large amount of literature is now accessible on the AMP mechanisms of action against Centers for Disease Control a diversity of pathogens; nevertheless, research on their activity on mycobacteria is still and Prevention, United States Kushagra Bansal, scarce. In particular, there is an urgent need to integrate all available interdisciplinary Harvard Medical School, strategies to eradicate extensively drug-resistant Mycobacterium tuberculosis strains. In United States this context, we should not underestimate our endogenous antimicrobial proteins and *Correspondence: peptides as ancient players of the human host defense system. We are confident that Ester Boix [email protected]; novel antibiotics based on human AMPs displaying a rapid and multifaceted mechanism, Sanjib Bhakta with reduced toxicity, should significantly contribute to reverse the tide of antimycobac- [email protected] terial drug resistance. -
Blood Lab First Week
Blood Lab First Week This is a self propelled powerpoint study atlas of blood cells encountered in examination of the peripheral blood smear. It is a requirement of this course that you begin review of these slides with the first day of class in order to familiarize yourself with terms used in describing peripheral blood morphology. Laboratory final exam questions are derived directly from these slides. The content of these slides may duplicate slides (1-37) listed on the Hematopathology Website. You must familiarize yourself with both sets of slides in order to have the best learning opportunity. Before Beginning This Slide Review • Please read the Laboratory information PDF under Laboratory Resources file to learn about – Preparation of a blood smear – How to select an area of the blood smear for review of morphology and how to perform a white blood cell differential – Platelet estimation – RBC morphology descriptors • Anisocytosis • Poikilocytosis • Hypochromia • Polychromatophilia Normal blood smear. Red blood cells display normal orange pink cytoplasm with central pallor 1/3-1/2 the diameter of the red cell. There is mild variation in size (anisocytosis) but no real variation in shape (poikilocytosis). To the left is a lymphocyte. To the right is a typical neutrophil with the usual 3 segmentations of the nucleus. Med. Utah pathology Normal blood: thin area Ref 2 Normal peripheral blood smear. This field is good for exam of cell morphology, although there are a few minor areas of overlap of red cells. Note that most cells are well dispersed and the normal red blood cell central pallor is noted throughout the smear. -

ORIGINAL ARTICLE PML–Rara Initiates Leukemia by Conferring Properties of Self-Renewal to Committed Promyelocytic Progenitors
Leukemia (2009) 23, 1462–1471 & 2009 Macmillan Publishers Limited All rights reserved 0887-6924/09 $32.00 www.nature.com/leu ORIGINAL ARTICLE PML–RARa initiates leukemia by conferring properties of self-renewal to committed promyelocytic progenitors S Wojiski1,2, FC Guibal3, T Kindler1,2, BH Lee1,2, JL Jesneck4,5, A Fabian1,2, DG Tenen3,6 and DG Gilliland1,2,6 1Division of Hematology, Brigham and Women’s Hospital, Boston, MA, USA; 2Department of Genetics, Howard Hughes Medical Institute, Harvard Medical School, Boston, MA, USA; 3Department of Hematology/Oncology, Harvard Institutes of Medicine, Boston, MA, USA; 4Cancer Program, Broad Institute of Harvard and MIT, Cambridge, MA, USA; 5Department of Pediatric Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA and 6Harvard Stem Cell Institute, Harvard University, Boston, MA, USA Acute promyelocytic leukemia (APL) is characterized by the leukemia stem cells (LSCs) are rare cells contained within hyperproliferation of promyelocytes, progenitors that are the more primitive CD34 þ CD38À population, resembling committed to terminal differentiation into granulocytes, making it an ideal disease in which to study the transforming potential transformed hematopoietic stem cells (HSCs). LSC activity was of less primitive cell types. We utilized a murine model of APL in shown by these investigators in all AML subtypes tested, but which the PML–RARa oncogene is expressed from the were not shown in acute promyelocytic leukemia (APL) endogenous cathepsin G promoter to test the hypothesis that associated with the expression of the PML–RARa fusion as a leukemia stem cell (LSC) activity resides within the differen- consequence of an acquired t(15;17).