Drini I Bardhë) River, Kosovo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogeography and Evolution of the Carassius Auratus-Complex in East
Takada et al. BMC Evolutionary Biology 2010, 10:7 http://www.biomedcentral.com/1471-2148/10/7 RESEARCH ARTICLE Open Access Biogeography and evolution of the Carassius auratus-complex in East Asia Mikumi Takada1,2*, Katsunori Tachihara1, Takeshi Kon2, Gunji Yamamoto2, Kei’ichiro Iguchi3, Masaki Miya4, Mutsumi Nishida2 Abstract Background: Carassius auratus is a primary freshwater fish with bisexual diploid and unisexual gynogenetic triploid lineages. It is distributed widely in Eurasia and is especially common in East Asia. Although several genetic studies have been conducted on C. auratus, they have not provided clear phylogenetic and evolutionary descriptions of this fish, probably due to selection bias in sampling sites and the DNA regions analysed. As the first step in clarifying the evolutionary entity of the world’s Carassius fishes, we attempted to clarify the phylogeny of C. auratus populations distributed in East Asia. Results: We conducted a detailed analysis of a large dataset of mitochondrial gene sequences [CR, 323 bp, 672 sequences (528 sequenced + 144 downloaded); CR + ND4 + ND5 + cyt b, 4669 bp in total, 53 sequences] obtained from C. auratus in East Asia. Our phylogeographic analysis revealed two superlineages, one distributed mainly among the Japanese main islands and the other in various regions in and around the Eurasian continent, including the Ryukyus and Taiwan. The two superlineages include seven lineages with high regional specificity that are composed of endemic populations indigenous to each region. The divergence time of the seven lineages was estimated to be 0.2 million years ago (Mya) by a fossil-based method and 1.0-1.9 Mya by the molecular clock method. -

Baseline Assessment of the Lake Ohrid Region - Albania
TOWARDS STRENGTHENED GOVERNANCE OF THE SHARED TRANSBOUNDARY NATURAL AND CULTURAL HERITAGE OF THE LAKE OHRID REGION Baseline Assessment of the Lake Ohrid region - Albania IUCN – ICOMOS joint draft report January 2016 Contents ........................................................................................................................................................................... i A. Executive Summary ................................................................................................................................... 1 B. The study area ........................................................................................................................................... 5 B.1 The physical environment ............................................................................................................. 5 B.2 The biotic environment ................................................................................................................. 7 B.3 Cultural Settings ............................................................................................................................ 0 C. Heritage values and resources/ attributes ................................................................................................ 6 C.1 Natural heritage values and resources ......................................................................................... 6 C.2 Cultural heritage values and resources....................................................................................... 12 D. -
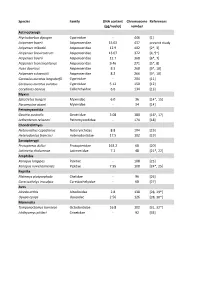
Table S2.Xlsx
Species Family DNA content Chromosome References (pg/nuclei) number Actinopterygii Ptychobarbus dipogon Cyprinidae ‐ 446 [1] Acipenser baerii Acipenseridae 15.02 437 present study Acipenser mikadoi Acipenseridae 12.9 402 [2*, 3] Acipenser brevirostrum Acipenseridae 13.07 372 [4, 5*] Acipenser baerii Acipenseridae 12.7 368 [6*, 7] Acipenser transmontanus Acipenseridae 9.46 271 [5*, 8] Huso dauricus Acipenseridae 8.3 268 [9*, 10] Acipenser schrenckii Acipenseridae 8.2 266 [9*, 10] Carassius auratus langsdorfii Cyprinidae ‐ 204 [11] Carassius auratus auratus Cyprinidae 5.12 150 [12] Corydoras aeneus Callichthyidae 6.6 134 [13] Myxini Eptatretus burgeri Myxinidae 6.0 36 [14*, 15] Paramyxine atami Myxinidae ‐ 34 [14] Petromyzontida Geotria australis Geotriidae 3.08 180 [16*, 17] Lethenteron reissneri Petromyzontidae ‐ 174 [18] Chondrichthyes Notorynchus cepedianus Notorynchidae 8.8 104 [19] Heterodontus francisci Heterodontidae 17.5 102 [19] Sarcopterygii Protopterus dolloi Protopteridae 163.2 68 [20] Latimeria chalumnae Latimeriidae 7.2 48 [21*, 22] Amphibia Xenopus longipes Pipidae ‐ 108 [23] Xenopus ruwenzoriensis Pipidae 7.95 108 [24*, 25] Reptilia Platemys platycephala Chelidae ‐ 96 [26] Carettochelys insculpta Carettochelyidae ‐ 68 [27] Aves Alcedo atthis Alcedinidae 2.8 138 [28, 29*] Upupa epops Upupidae 2.56 126 [28, 30*] Mammalia Tympanoctomys barrerae Octodontidae 16.8 102 [31, 32*] Ichthyomys pittieri Cricetidae ‐ 92 [33] References 1. Yu XY, Yu XJ. A schizothoracin fish species, Diptychus dipogon, with very high number of chromosomes Chrom Inform Serv. 1990;48:17-8. 2. Zhou H, Fujimoto T, Adachi S, Yamaha E, Arai K. Genome size variation estimated by flow cytometry in Acipenser mikadoi, Huso dauricus in relation to other species of Acipenseriformes. -

A-Bohlen.Vp:Corelventura
Folia biologica (Kraków), vol. 51 (2003), Supplement Cobitis ohridana and Barbatula zetensis in the River Moraèa Basin, Montenegro: distribution, Habitat, Population Structure and Conservation Needs Jörg BOHLEN, Vendula ŠLECHTOVÁ, Radek ŠANDA, Jörg FREYHOF, Jasna VUKIC, and Danilo MRDAK Accepted April 17, 2003 BOHLEN J., ŠLECHTOVÁ V., ŠANDA R., KALOUS L., FREYHOF J., VUKIC J., MRDAK D. 2003. Cobitis ohridana and Barbatula zetensis in the River Moraèa basin, Montenegro: distribution, habitat, population structure and conservation needs. Folia biol. (Kraków) 51(Suppl.): 147-153. In this paper, we report on the distribution, habitat, population structure and conservation needs of Cobitis ohridana and Barbatula zetensis in the basin of the River Moraèa in Montenegro. Our data show both species to be mainly distributed in the lower stretch of the main river and some tributaries in lowland habitats. Cobitis ohridana preferred more shallow water with a higher abundance of filamentous algae, while Barbatula zetensis was more numerous in slightly deeper water with more stones as a bottom substrate. Slight differences in the habitat preference were also observed between juveniles and adults in both species. Although both species are abundant in suited habitat, they have a small distribution area in the Moraèa basin due to the natural rarity of the habitat. According to our data, they are not endangered. Key words: Balitoridae, Cobitidae, Mediterranean, conservation, habitat preferences, outecology. Jörg BOHLEN, Vendula ŠLECHTOVÁ, Lukáš KALOUS,Institute of Animal Physiology and Ge- netics, Academy of Sciences of the Czech Republic, 277 21 Libechov, Czech Republic. E-mail: [email protected] Radek ŠANDA, Charles University, Faculty of Science, Department of Zoology, Vinièná 7, 128 44 Prague, Czech Republic; Czech National Museum, Department of Zoology, Václavské námìstí 68, 115 79 Prague, Czech Republic. -

Drin River Basin the Blue Heart of the Balkans
DDrriinn RRiivveerr BBaassiinn TThhee bblluuee hheeaarrtt ooff tthhee BBaallkkaannss 1 Drin River Basin: the bleu heart of the Balkans The Mediterranean Information Office for © MIO‐ECSDE 2012 Kyrristou 12, 10556 Athens, Greece Environment, Culture and Sustainable Tel: +30210‐3247490, ‐3247267, Fax: +30210 3317127 Development (MIO‐ECSDE) is a non‐profit e‐mail: info@mio‐ecsde.org Federation of 126 Mediterranean NGOs for Environment and Development. MIO‐ECSDE This publication has been produced within the acts as a technical and political platform for framework of the DG Environment programme for the presentation of views and intervention operating grants to European environmental NGOs. of NGOs in the Mediterranean scene and plays an active role for the protection of the Written/prepared by: environment and the promotion of the Thomais Vlachogianni, Milan Vogrin sustainable development of the Text editing: Mediterranean region and its countries. Anastasia Roniotes, MIO‐ECSDE Head Officer Website: www.mio‐ecsde.org This publication is available on line at www.mio‐ ecsde.org Contents Drin River Basin: the blue heart of the Balkans ...................................................................................... 3 The Drin River: the ‘connecting body’ of a water system that forms an eco‐region of global significance .............................................................................................................................................. 3 Drin River Basin: an exceptional wealth of habitats and species ........................................................... -

Barbatula Leoparda (Actinopterygii, Nemacheilidae), a New Endemic Species of Stone Loach of French Catalonia
Scientific paper Barbatula leoparda (Actinopterygii, Nemacheilidae), a new endemic species of stone loach of French Catalonia by Camille GAULIARD (1), Agnès DETTAI (2), Henri PERSAT (1, 3), Philippe KEITH (1) & Gaël P.J. DENYS* (1, 4) Abstract. – This study described a new stone loach species in France, Barbatula leoparda, which is endemic to French Catalonia (Têt and Tech river drainages). Seven specimens were compared to 49 specimens of B. bar- batula (Linnaeus, 1758) and 71 specimens of B. quignardi (Băcescu-Meşter, 1967). This new species is char- acterized by the presence of blotches on the belly and the jugular area in individuals longer than 47 mm SL and by a greater interorbital distance (35.5 to 41.8% of the head length). We brought moreover the sequence of two mitochondrial markers (COI and 12S, respectively 652 and 950 bp) of the holotype, which are well distinct from all other species, for molecular identifications. This discovery is important for conservation. Résumé. – Barbatula leoparda (Actinopterigii, Nemacheilidae), une nouvelle espèce endémique de loche fran- che en Catalogne française. © SFI Submitted: 4 Jun. 2018 Cette étude décrit une nouvelle espèce de loche franche en France, Barbatula leoparda, qui est endémique Accepted: 23 Jan. 2019 Editor: G. Duhamel à la Catalogne française (bassins de la Têt et du Tech). Sept spécimens ont été comparés à 49 spécimens de B. barbatula (Linnaeus, 1758) et 71 spécimens de B. quignardi (Băcescu-Meşter, 1967). Cette nouvelle espèce est caractérisée par la présence de taches sur le ventre et dans la partie jugulaire pour les individus d’une taille supérieure à 47 mm LS et par une plus grande distance inter-orbitaire (35,5 to 41,8% de la longueur de la tête). -

Hydrology of the Drini River Basin, Albania
University of Texas at Austin GIS in Water Resources Instructor: Dr. David Maidment HYDROLOGY OF THE TRANSBOUNDARY DRIN RIVER BASIN Wikipedia Elisabeta Poci December, 2011 1 Table of Contents: 1. Introduction and Background 2. Watershed Delineation 3. Volume of Water for Run-Off 4. Results and Conclussions 5. Literature List of Figures: Figure 1. Location of study area Figure 2. Rivers and Lakes part of the Drin Basin Figure 3. Prespa Lakes Figure 4. Ohrid Lake looking South at Inflow from Prespa Figure 5. Data Download from the Hydrosheds site for our area of interest Figure 6. World’s Watersheds shape file (15sec DEM) Figure 7. Flow Direction shape file (3sec DEM) Figure 8. Drini Basin exported and saved as a New Feature Class Figure 9. Drainage Direction DEM clipped with the Drin Basin Figure 10. Isolated Watershed with the Outlet Point Figure 11. Projecting the raster Figure 12. Raster Calculator Formula Figure 13. The delineated Drin River Basin Figure 14. Area of the Basin Figure 15. Comparison of my map with the map found on the web. Extension of the Basin towards the Prespa Lakes Figure 16. Zooming in to the Prespa Lakes Figure 17. Shapefile of Countries Projected and Clipped Figure 18. Intersected Shape files of Countries with Catchments Figure 19. Attributes table of the Intersected shape file Figure 20. Precipitation raster opened in ArcGIS Figure 21. Downloading precipitation data from the website of GPCC Figure 22. Model used for Precipitation Raster Figure 23. Clipped Precipitation Rater and Zonal Statistics as Table Figure 24. Volume for Run Off (km 3) Figure 25. -

Albania Environmental Performance Reviews
Albania Environmental Performance Reviews Third Review ECE/CEP/183 UNITED NATIONS ECONOMIC COMMISSION FOR EUROPE ENVIRONMENTAL PERFORMANCE REVIEWS ALBANIA Third Review UNITED NATIONS New York and Geneva, 2018 Environmental Performance Reviews Series No. 47 NOTE Symbols of United Nations documents are composed of capital letters combined with figures. Mention of such a symbol indicates a reference to a United Nations document. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. In particular, the boundaries shown on the maps do not imply official endorsement or acceptance by the United Nations. The United Nations issued the second Environmental Performance Review of Albania (Environmental Performance Reviews Series No. 36) in 2012. This volume is issued in English only. Information cut-off date: 16 November 2017. ECE Information Unit Tel.: +41 (0)22 917 44 44 Palais des Nations Fax: +41 (0)22 917 05 05 CH-1211 Geneva 10 Email: [email protected] Switzerland Website: http://www.unece.org ECE/CEP/183 UNITED NATIONS PUBLICATION Sales No.: E.18.II.E.20 ISBN: 978-92-1-117167-9 eISBN: 978-92-1-045180-2 ISSN 1020–4563 iii Foreword The United Nations Economic Commission for Europe (ECE) Environmental Performance Review (EPR) Programme provides assistance to member States by regularly assessing their environmental performance. Countries then take steps to improve their environmental management, integrate environmental considerations into economic sectors, increase the availability of information to the public and promote information exchange with other countries on policies and experiences. -
Fishes of the River Vjosa – an Annotated Checklist
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/329238572 The Vjosa catchment – a natural heritage Article · November 2018 CITATIONS READS 12 353 9 authors, including: Spase Shumka Sajmir Beqiraj Agricultural University of Tirana University of Tirana 197 PUBLICATIONS 621 CITATIONS 48 PUBLICATIONS 600 CITATIONS SEE PROFILE SEE PROFILE Anila Paparisto Lefter Kashta University of Tirana University of Tirana 46 PUBLICATIONS 113 CITATIONS 47 PUBLICATIONS 418 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Assesment of rare and endangered plant species, and invertebrates and their habitats in the mountainous areas of Korça, Berati and Vlora districts View project Climate changes adaption interventions of the Kune-Vaini lagoon system (Lezha, Albania) - ecological approach View project All content following this page was uploaded by Aleko Miho on 11 March 2019. The user has requested enhancement of the downloaded file. Acta ZooBot Austria 155, 2018, 163–176 Fishes of the River Vjosa – an annotated Checklist Spase Shumka, Paul Meulenbroek, Fritz Schiemer & Radek Šanda Based on a combination of intensive fieldwork for a period of thirteen years (2004– 2017), literature review and review of museum specimens, we hereby provide an up- dated checklist of the fishes of Albanian part of River Vjosa. Our results show that there are at least 31 species of fishes inhabiting the river system, of which 27 are native, including eight species endemic to the Balkans. With 11 species, Cyprinidae are by far the most specious family, followed by Mugilidae (five). Salmonidae and Acipenseridae are represented by 2 species each. -

Mapping of Solid Waste in the Rivers of the Municiplity of Kukës
SOLID WASTE MAPPING IN KUKES’ RIVERS 2 MAPPING OF SOLID WASTE IN THE RIVERS OF THE MUNICIPALITY OF KUKES SOLID WASTE MAPPING IN KUKES’ RIVERS 3 This publication is supported by the 2014-2020 IPA Cross-Border Program Albania-Kosovo, funded by the European Union and managed by the European Commission Delegation in Albania. This document was drafted as part of the “Gjakova and Kukes Clean Water Project” which is being implemented after the 2nd round of calls for project proposals of the IPA Cross-Border Program between Albania and Kosovo throughout 2014 - 2020, funded by the European Union and managed by the European Commission Delegation in Albania. The Clean Water Project in Gjakova and Kukes is being implemented by a consortium of non- governmental actors and local governments, under the direction of SHE-ERA. Other members of the consortium include the Municipality of Gjakova, the Municipality of Kukes and, the Albanian Center for Economic Research (ACER). The research on mapping the waste and landfills in rivers in the municipality of Gjakova in Kosovo and the municipality of Kukes in Albania was conducted by the Non-Governmental Organization Let’s Do It Peja between the 10th of October, 2020, and 24th of January, 2021. Address: Women Business Association SHE-ERA “Jakova Innovation Center” First Floor - “Gjakova and Kukes Clean Water Project” St. Sylejman Hadum Aga, #189, Email address: [email protected] SOLID WASTE MAPPING IN KUKES’ RIVERS 4 Table of Contents 1. INTRODUCTION ............................................................................................................................... 6 2. METHODOLOGY .............................................................................................................................. 7 3. INFORMATION ON THE MUNICIPALITY OF KUKES .......................................................................... 9 4. LEGISLATION ON WATER PROTECTION AND TREATMENT .......................................................... -

Drin Project
Call for Expressions of Interest: Final evaluation of the project ‘Design and testing of a multipurpose (transboundary) groundwater monitoring network (Albania & Montenegro)” (Submission deadline: 28 October 2020) The Antenna in Sarajevo of the UNESCO Regional Bureau for Science and Culture in Europe, is seeking expressions of interest from qualified and experienced individuals, to carry out the final evaluation of the project entitled ‘Design and testing of a multipurpose (transboundary) groundwater monitoring network (Albania and Montenegro)” I. Background Brief description of the project: 1.1 Drin Project Setting out from the two Prespa Lakes, linked to each other by a small channel, water flows through underground karst cavities to Lake Ohrid, the largest lake in terms of water volume in South-East Europe. The only surface outflow of Lake Ohrid, the Black Drin River flows north through the Republic of North Macedonia and enters Albania. The White Drin River flows into Albania, where it meets the Black Drin and forms the Drin River. Flowing westward through Albania, the Drin River meets the Buna/Bojana River, close after the outflow of the latter from Lake Skadar/Shkoder, the largest lake in terms of surface in South-East Europe. The Buna/Bojana River directly discharges into the Adriatic Sea. The overall concept for enhanced cooperation among the Riparians for the management of the Basin was initially discussed by representatives of the competent ministries and other key stakeholders during the International Roundtable on Integrated Management of Shared Lake Basins in South-Eastern Europe, organized under the Petersberg Phase II/Athens Declaration Process and the Global Environment Facility (GEF) IW:LEARN Programme, in Ohrid, on 12- 14 October 2006. -

5Th Indo-Pacific Fish Conference
)tn Judo - Pacifi~ Fish Conference oun a - e II denia ( vernb ~ 3 - t 1997 A ST ACTS Organized by Under the aegis of L'Institut français Société de recherche scientifique Française pour le développement d'Ichtyologie en coopération ' FI Fish Conference Nouméa - New Caledonia November 3 - 8 th, 1997 ABSTRACTS LATE ARRIVAL ZOOLOGICAL CATALOG OF AUSTRALIAN FISHES HOESE D.F., PAXTON J. & G. ALLEN Australian Museum, Sydney, Australia Currently over 4000 species of fishes are known from Australia. An analysis ofdistribution patterns of 3800 species is presented. Over 20% of the species are endemic to Australia, with endemic species occuiring primarily in southern Australia. There is also a small component of the fauna which is found only in the southwestern Pacific (New Caledonia, Lord Howe Island, Norfolk Island and New Zealand). The majority of the other species are widely distributed in the western Pacific Ocean. AGE AND GROWTH OF TROPICAL TUNAS FROM THE WESTERN CENTRAL PACIFIC OCEAN, AS INDICATED BY DAILY GROWm INCREMENTS AND TAGGING DATA. LEROY B. South Pacific Commission, Nouméa, New Caledonia The Oceanic Fisheries Programme of the South Pacific Commission is currently pursuing a research project on age and growth of two tropical tuna species, yellowfm tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus). The daily periodicity of microincrements forrned with the sagittal otoliths of these two spceies has been validated by oxytetracycline marking in previous studies. These validation studies have come from fishes within three regions of the Pacific (eastem, central and western tropical Pacific). Otolith microincrements are counted along transverse section with a light microscope.