The Sacroiliac Part of the Iliolumbar Ligament
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basic Biomechanics
Posture analysis • A quick evaluation of structure and function • Doctor views patient from behind (P-A) and from the side (lateral) • References points of patient’s anatomy relative to plumb (a position of balance) 9/3/2013 1 Posture analysis • Lateral View – Knees (anterior, posterior, plumb, genu recurvatum) – Trochanter (anterior, posterior, plumb) – Pelvis (anterior, posterior, neutral pelvic tilt) – Lumbar lordosis (hypo-, hyper-, normal) – Mid-axillary line (anterior, posterior, plumb) – Thoracic kyphosis (hyp-, hyper- normal) – Acromion (anterior, posterior, plumb) – Scapulae (protracted, retracted, normal) – Cervical lordosis (hypo-, hyper-, normal) – External auditory meatus (anterior, posterior, plumb) – Occiput (extended, neutral, flexed) 9/3/2013 2 Posture analysis • Posterior – Anterior View – Feet (pronation, supination, normal) – Achilles tendon (bowed in/out, normal) – Knees (genu valga/vera, normal - internal/external rotation) – Popliteal crease heights (low, high, level) – Trochanter heights (low, high, level) – Iliac crest heights (low on the right/left, normal) – Lumbar scoliosis (right/left, or no signs of) – Thoracic scoliosis (right/left, or no signs of) – Shoulder level (low on the right/left, or normal) – Cervical scoliosis (right/left, or no signs of) – Cervical position (rotation, tilt, neutral) – Mastoid (low on the right/left, or normal) 9/3/2013 3 …..poor postures 9/3/2013 4 Functional Anatomy of the Spine • The vertebral curvatures – Cervical Curve • Anterior convex curve (lordosis) develop in infancy -

Applied Anatomy of the Hip RICARDO A
Applied Anatomy of the Hip RICARDO A. FERNANDEZ, MHS, PT, OCS, CSCS • Northwestern University The hip joint is more than just a ball-and- bones fuse in adults to form the easily recog- socket joint. It supports the weight of the nized “hip” bone. The pelvis, meaning bowl head, arms, and trunk, and it is the primary in Latin, is composed of three structures: the joint that distributes the forces between the innominates, the sacrum, and the coccyx pelvis and lower extremities.1 This joint is (Figure 1). formed from the articu- The ilium has a large flare, or iliac crest, Key PointsPoints lation of the proximal superiorly, with the easily palpable anterior femur with the innomi- superior iliac spine (ASIS) anterior with the The hip joint is structurally composed of nate at the acetabulum. anterior inferior iliac spine (AIIS) just inferior strong ligamentous and capsular compo- The joint is considered to it. Posteriorly, the crest of the ilium ends nents. important because it to form the posterior superior iliac spine can affect the spine and (PSIS). With respect to surface anatomy, Postural alignment of the bones and joints pelvis proximally and the PSIS is often marked as a dimple in the of the hip plays a role in determining the femur and patella skin. Clinicians attempting to identify pelvic functional gait patterns and forces associ- distally. The biomechan- or hip subluxations, leg-length discrepancies, ated with various supporting structures. ics of this joint are often or postural faults during examinations use There is a relationship between the hip misunderstood, and the these landmarks. -

How to Perform a Transrectal Ultrasound Examination of the Lumbosacral and Sacroiliac Joints
DIAGNOSTIC IMAGING How to Perform a Transrectal Ultrasound Examination of the Lumbosacral and Sacroiliac Joints Erik H.J. Bergman, DVM, Diplomate ECAR, Associate Member LA-ECVDI*; Sarah M. Puchalski, DVM, Diplomate ACVR; and Jean-Marie Denoix, DVM, PhD, Agre´ge´, Associate Member LA-ECVDI Authors’ addresses: Lingehoeve Veldstraat 3 Lienden 4033 AK, The Netherlands (Bergman); Uni- versity of California, Davis, One Shields Avenue, School of Veterinary Medicine, Davis, CA 95616 (Puchalski); E´ cole Nationale Ve´te´rinaire d’Alfort, 7 Avenue du Ge´ne´ral de Gaulle, 94700 Maisons- Alfort, France (Denoix); e-mail: [email protected]. *Corresponding and presenting author. © 2013 AAEP. 1. Introduction have allowed for identification of these structures 5 There is increasing interest in pathology of the and the inter-transverse joints. These authors urge lumbosacral and sacroiliac joints giving rise to stiff- caution in the interpretation of lesions identified on ness and/or lameness and decreased performance radiography in the absence of other diagnostic im- in equine sports medicine.1–3 Pain arising from aging and clinical examination. Nuclear scintigra- these regions can be problematic alone or in con- phy is an important component of work-up for junction with lameness arising from other sites sacroiliac region pain, but limitations exist. Sev- 9,10 (thoracolumbar spine, hind limbs, or forelimbs).4 eral reports exist detailing the anatomy and tech- Localization of pain to this region is critically impor- nique findings in normal horses11,12 and findings in tant through clinical assessment, diagnostic anes- lame horses.13 Patient motion, camera positioning, thesia, and imaging. and muscle asymmetry can cause errors in interpre- In general, diagnostic imaging of the axial skele- tation. -

Between Ligamentous Structures in the Thoracic Spine : a finite Element Investigation
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Queensland University of Technology ePrints Archive This is the author’s version of a work that was submitted/accepted for pub- lication in the following source: Little, J. Paige & Adam, Clayton J. (2011) Effects of surgical joint desta- bilization on load sharing between ligamentous structures in the thoracic spine : a finite element investigation. Clinical Biomechanics, 26(9), pp. 895-903. This file was downloaded from: http://eprints.qut.edu.au/48159/ c Copyright 2011 Elsevier Ltd. NOTICE: this is the author’s version of a work that was accepted for publication in the journal Clinical Biomechanics. Changes resulting from the publishing process, such as peer review, editing, corrections, struc- tural formatting, and other quality control mechanisms may not be re- flected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was sub- sequently published in Clinical Biomechanics 26 (2011) 895–903, DOI: 10.1016/j.clinbiomech.2011.05.004 Notice: Changes introduced as a result of publishing processes such as copy-editing and formatting may not be reflected in this document. For a definitive version of this work, please refer to the published source: http://dx.doi.org/10.1016/j.clinbiomech.2011.05.004 *Manuscript (including title page & abstract) Effect of surgical joint destabilization on ligament load-sharing 1 Effects of surgical joint destabilization on load sharing 2 between ligamentous structures in the thoracic spine: A 3 Finite Element investigation 4 5 Authors: 6 J. -

Epithelia Joitns
NAME LOCATION STRUCTURE FUNCTION MOVEMENT Temporomandibular joint Condylar head of ramus of Synovial Diarthrosis Modified hinge joint mandible and glenoid fossa of Rotation and gliding temporal bone Biaxial Zygapophyseal joint Between articular processes of Synovial Diarthrosis Gliding 2 adjacent vertebrae Non axial Atlanto-Occipital joints Atlas and occipital condyle of Synovial Diarthrosis Ellipsoid occipital bone Biaxial Atlantoaxial joints Atlas and axis Synovial Diarthrosis Pivot Uniaxial Joints of vertebral arches Ligaments Fibrous Amphiarthrosis Syndesmoses Intervertebral symphyseal Intervertebral disk between 2 Cartilaginous Amphiarthrosis joints vertebrae Symphysis Costovertebral Head of ribs and body of Synovial Diarthrosis Gliding thoracic vertebra Non axial Costotrasnverse joints Tubercle of rib and transverse Synovial Diarthrosis Gliding process of thoracic vertebra Non axial Lumbosacral Joint Left and right zygopophyseal Laterally Synovial joint Intervertebral symphyseal joint Symphysis SternoclavicularJoint Clavicular notch articulates Synovial Diarthrosis Gliding with medial ends of clavicle Non Axial Manubriosternal Joint Hyaline cartilage junction Cartilaginous Synarthrosis Sternal Angle between manubrium and body Symphysis Xiphisternal Joint Cartilage between xiphoid Synchondrosis Synarthrosis process and body Synostoses Sternocostal Joint (1st) Costocartilage 1 with sternum Cartilaginous Synchondrosis Synarthrosis NAME Location Section Anterior longitudinal runs down anterior surface of vertebral body Vertebral column ligament Posterior longitudinal in canal, runs down posterior surface of vertebral body ligament Interspinous ligament Connects spinous processes Ligamentum flavum Connects laminae ! Intra-articular Disc Between articulating surface of sternum and clavicle Sternoclavicular Joint Costoclavicular ligament 1st rib to clavicle !. -

Canine Thoracic Costovertebral and Costotransverse Joints Three Case Reports of Dysfunction and Manual Therapy Guidelines for A
Topics in Compan An Med 29 (2014) 1–5 Topical review Canine Thoracic Costovertebral and Costotransverse Joints: Three Case Reports of Dysfunction and Manual Therapy Guidelines for Assessment and Treatment of These Structures Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRTn Keywords: The costovertebral and costotransverse joints receive little attention in research. However, pain costovertebral associated with rib articulation dysfunction is reported to occur in human patients. The anatomic costotransverse structures of the canine rib joints and thoracic spine are similar to those of humans. As such, it is ribs physical therapy proposed that extrapolation from human physical therapy practice could be used for the assessment and rehabilitation treatment of the canine patient with presumed rib joint pain. This article presents 3 case studies that manual therapy demonstrate signs of rib dysfunction and successful treatment using primarily physical therapy manual techniques. General assessment and select treatment techniques are described. & 2014 Elsevier Inc. All rights reserved. The Canine Fitness Centre Ltd, Calgary, Alberta, Canada nAddress reprint requests to Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRT, The Canine Fitness Centre Ltd, 509—42nd Ave SE, Calgary, Alberta, Canada T2G 1Y7 E-mail: [email protected] The articular structures of the thorax comprise facet joints, the erect spine and further presented that in reviewing the literature, intervertebral disc, and costal joints. Little research has been they were unable to find mention of natural development of conducted on these joints in human or animal medicine. However, idiopathic scoliosis in quadrupeds; however, there are reports of clinical case presentations in human journals, manual therapy avian models and adolescent models in man. -

Acetabular Center Axis: Is It the Future of Hip Navigation?
■ Feature Article Acetabular Center Axis: Is It the Future of Hip Navigation? SAM HAKKI, MD; VICTOR BILOTTA, MD; J. DANIEL OLIVEIRA, MD; LUIS DORDELLY, BS abstract There are 2 distinct methods of cup navigation in total hip arthroplasty. One resorted to piercing the skin to improve predicts orientation of the acetabulum through bony landmarks outside the ac- their registration efforts; others resorted etabulum (eg, the anterior pelvic plane); its unreliability is well published. The to using “adjusted” APP registration other identifi es acetabular center axis (ACA) and is patient-specifi c method that in which the APP registration is calcu- is independent of pelvic tilt, making it more reliable. Data from readily pal- lated according to the change of APP pable acetabular registration points were compared with postoperative pelvic with changes of pelvic tilt. Some results computed tomography images in 137 cases. Findings show that ACA software showed fairly accurate registration and is accurate in determining acetabular/cup version and inclination. Cup center good prediction of the inclination angle axis should coincide within 4 mm of ACA to minimize impingement and maxi- and version of the acetabulum.16-18 How- mize stability without altering preoperative femoral version. ever, the new hip center could be cranial, caudal, anterior, or posterior in relation to the acetabular center, or it could be he femoral neck axis normally device (eg, a Tilt-Meter) or by imaging, medialized by implant design or to gain coincides with the acetabu- such -

Bones and Joints of the Lower Limb: Pelvic Girdle and Femur
Unit 5: Bones and joints of the lower limb: pelvic girdle and femur Chapter 5 (Lower limb) and Chapter 3 (Pelvis and perineum) GENERAL OBJECTIVES: - recognize, name and correctly orient hip bones and femur - explain how is anatomy of hip bones/pelvis adjusted to its function - name and describe all joints of pelvis focusing of anatomical and functional properties - remember concepts and common structural properties of flat and long bones SPECIFIC OBJECTIVES: Bones of the pelvic girdle and femur HIP BONE Describe anatomical position of the hip bone, which bony elements lay in frontal plane? Which primary bones fuse to form hip bone? What are differences between male and female pelvis? Identify the bony structures on each of the following parts of the HIP BONE. Ileum: the body and alae, - Iliac crest - Gluteal surface and lines - Iliac fossa - Sacral side with auricular surface and iliac tuberosity Pubis: the body and rami (superior and inferior) - Superior ramus - Inferior ramus Ischium: the body and ramus -Ischial spine and tuberostiy -Greater and lesser sciatic notches Acetebulum Obturator foramen FEMUR - Upper (proximal) end: head, neck, angles, trochanters, intertrochanteric crest, trochanteric fossa - Shaft: linea aspera with lips - Lower (distal) end: condyles, intercondilar fossa, patellar surface, Joints of the pelvis and hip Bony Pelvis (Hip Bones, Sacrum & Coccyx) Bony Features & Articular Surfaces Attachments of: Ligaments & Muscles Lesser Pelvis Pelvic Brim -> Pelvic Inlet (Superior Aperture) Lateral & Posterior Walls: Obturator -
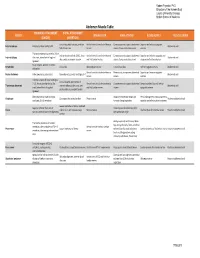
Abdomen Muscle Table PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION)
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine Abdomen Muscle Table PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Linea alba, pubic tubercle, anterior Ventral rami of six inferior thoracic Compresses and supports abdominal Superior and inferior epigastric External oblique External surfaces of ribs 5–12 Abdominal wall half of iliac crest nerves viscera, flexes and rotates trunk arteries Thoracolumbar fascia, anterior 2/3 of Inferior borders of ribs 10–12, linea Ventral rami of six inferior thoracic Compresses and supports abdominal Superior and inferior epigastric and Internal oblique iliac crest, lateral half of inguinal Abdominal wall alba, pubis via conjoint tendon and first lumbar nerves viscera, flexes and rotates trunk deep circumflex iliac arteries ligament Body of pubis, anterior to rectus Pyramidalis Linea alba Iliohypogastric nerve Tenses linea alba Inferior epigastric artery Abdominal wall abdominis Ventral rami of six inferior thoracic Flexes trunk, compresses abdominal Superior and interior epigastric Rectus abdominis Pubic symphysis, pubic crest Xiphoid process, costal cartilages 5–7 Abdominal wall nerves viscera arteries Internal surfaces of costal cartilages Linea alba with aponeurosis of 7–12, thoracolumbar fascia, iliac Ventral rami of six inferior thoracic Compresses and supports abdominal Deep circumflex iliac and inferior Transversus abdominis internal oblique, pubic crest, and Abdominal wall -

The Lumbosacral Dorsal Rami of the Cat
J. Anat. (1976), 122, 3, pp. 653-662 653 With 1O figures Printed in Great Britain The lumbosacral dorsal rami of the cat NIKOLAI BOGDUK Department ofAnatomy, University ofSydney, Sydney, Australia (Accepted 2 December 1975) INTRODUCTION Several reflexes involving dorsal rami have been demonstrated in the cat (Pedersen, Blunck & Gardner, 1956; Bogduk & Munro, 1973). However, there is no adequate description in the literature of the anatomy of lumbosacral dorsal rami in this animal. The present study was therefore undertaken to provide such a description, hoping thereby to facilitate the design and interpretation of our own (Bogduk & Munro, 1973) and future research on reflexes involving lumbosacral dorsal rami, including reflexes possibly relevant to the understanding of back pain in man. These nerves are described in the present study in relation to a revised nomen- clature of the muscles in the dorsal lumbar region. Such a revision (Bogduk, 1975) was necessary because of the different nomenclatures and varied interpretations in the literature. METHODS Six laboratory cats (Felis domesticus) were embalmed with 10° formalin and studied by gross dissection. In addition, confirmatory observations were made on another 16 cats in the course of surgical procedures. Lateral branches of dorsal rami were first identified during reflexion of the skin and then during the resection of iliocostalis and longissimus lumborum. These branches were subsequently traced back to their origins from the dorsal rami, a dissecting microscope being used. The medial branches of the dorsal rami were then traced through the intertransversarii mediales into multifidus. Sinuvertebral nerves were also sought. Nerve roots were detached from the spinal cord before removing it from the vertebral canal. -

Biomechanics of the Sacroiliac Joint: Part I−−Anatomy, Function, Biomechanics, Sexual Dimorphism, and Causes of Pain
Biomechanics of the Sacroiliac Joint: Part I−−Anatomy, Function, Biomechanics, Sexual Dimorphism, and Causes of Pain Ali Kiapour, Amin Joukar, Hossein Elgafy, Deniz U. Erbulut, Anand K. Agarwal and Vijay K. Goel Int J Spine Surg published online 30 December 2019 http://ijssurgery.com/content/early/2019/12/30/6077 This information is current as of September 23, 2021. Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://ijssurgery.com/alerts The International Journal of Spine Surgery 2397 Waterbury Circle, Suite 1, Aurora, IL 60504, Phone: +1-630-375-1432 © 2019 ISASS. All RightsDownloaded Reserved. from http://ijssurgery.com/ by guest on September 23, 2021 International Journal of Spine Surgery, Vol. 00, No. 00, 0000, pp. 000–000 https://doi.org/10.14444/6077 ÓInternational Society for the Advancement of Spine Surgery Biomechanics of the Sacroiliac Joint: Part I—Anatomy, Function, Biomechanics, Sexual Dimorphism, and Causes of Pain ALI KIAPOUR, PHD,1,2 AMIN JOUKAR, MS,1 HOSSEIN ELGAFY, MD,1 DENIZ U. ERBULUT, PHD,1 ANAND K. AGARWAL, MD,1 VIJAY K. GOEL, PHD1 1Engineering Center for Orthopaedic Research Excellence (E-CORE), Departments of Bioengineering and Orthopaedics, The University of Toledo, Toledo, Ohio; 2Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts ABSTRACT Background: The sacroiliac joints (SIJs), the largest axial joints in the body, sit in between the sacrum and pelvic bones on either side. They connect the spine to the pelvis and thus facilitate load transfer from the lumbar spine to the lower extremities. The majority of low back pain (LBP) is perceived to originate from the lumbar spine; however, another likely source of LBP that is mostly overlooked is the SIJ. -

Effects of Different Angles of the Traction Table on Lumbar Spine Ligaments: a Finite Element Study Hekmat Farajpour, MS, Nima Jamshidi, Phd
Original Article Clinics in Orthopedic Surgery 2017;9:480-488 • https://doi.org/10.4055/cios.2017.9.4.480 Effects of Different Angles of the Traction Table on Lumbar Spine Ligaments: A Finite Element Study Hekmat Farajpour, MS, Nima Jamshidi, PhD Department of Biomedical Engineering, Faculty of Engineering, University of Isfahan, Isfahan, Iran Background: The traction bed is a noninvasive device for treating lower back pain caused by herniated intervertebral discs. In this study, we investigated the impact of the traction bed on the lower back as a means of increasing the disc height and creating a gap between facet joints. Methods: Computed tomography (CT) images were obtained from a female volunteer and a three-dimensional (3D) model was cre- ated using software package MIMICs 17.0. Afterwards, the 3D model was analyzed in an analytical software (Abaqus 6.14). The study was conducted under the following traction loads: 25%, 45%, 55%, and 85% of the whole body weight in different angles. Results: Results indicated that the loading angle in the L3–4 area had 36.8%, 57.4%, 55.32%, 49.8%, and 52.15% effect on the anterior longitudinal ligament, posterior longitudinal ligament, intertransverse ligament, interspinous ligament, and supraspinous ligament, respectively. The respective values for the L4–5 area were 32.3%, 10.6%, 53.4%, 56.58%, and 57.35%. Also, the body weight had 63.2%, 42.6%, 44.68%, 50.2%, and 47.85% effect on the anterior longitudinal ligament, posterior longitudinal liga- ment, intertransverse ligament, interspinous ligament, and supraspinous ligament, respectively. The respective values for the L4–5 area were 67.7%, 89.4%, 46.6%, 43.42% and 42.65%.