Prevalence and Parasitic Burden in Mollusc Intermediate Hosts From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Universidade Federal De Juiz De Fora Pós-Graduação Em Ciências Biológicas Mestrado Em Comportamento E Biologia Animal
UNIVERSIDADE FEDERAL DE JUIZ DE FORA PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS MESTRADO EM COMPORTAMENTO E BIOLOGIA ANIMAL Camilla Aparecida de Oliveira Estratégia de história de vida e recaracterização morfológica Sarasinula linguaeformis (Semper, 1885) (Eupulmonata, Veronicellidae) Juiz de Fora 2019 Camilla Aparecida de Oliveira Estratégia de história de vida e recaracterização morfológica Sarasinula linguaeformis (Semper, 1885) (Eupulmonata, Veronicellidae) Dissertação apresentada ao Programa de Pós-Graduação em Ciências Biológicas, área de concentração: Comportamento e Biologia Animal da Universidade Federal de Juiz de Fora, como requisito parcial para obtenção do título de Mestre. Orientadora: Prof.ª. Drª. Sthefane D’ávila Juiz de Fora 2019 A todos que estiveram ao meu lado me apoiando e incentivando diante das dificuldades da carreira acadêmica, e incentivaram minha formação pessoal, profissional e dando-me suporte emocional. A vocês o meu eterno agradecimento! AGRADECIMENTOS Agradeço primeiramente a Deus por abençoar o meu caminho durante esse trabalho. A fé que tenho em Ti alimentou meu foco, minha força e minha disciplina. Depois aos meus amigos da Ciências Biológicas: Alexssandra Silva, Flávio Macanha, Isabel Macedo, Sue-helen Mondaini, Tayrine Carvalho, Kássia Malta e Yuri Carvalho meu eterno agradecimento, pois fizeram uma contribuição valiosa para a minha jornada acadêmica com seus conselhos, auxílio, palavras de apoio e risadas. Também agradeço a todos aqueles amigos que de forma direta ou indireta estiveram ajudando e torcendo por mim, em especial a Ana Claudia Mazetto, Ana Clara Files, Tamires Lima, Lígia Araújo, Raquel Seixas, Natália Corrêa e Carlota Augusta. Vocês foram fundamentais para minha formação. Agradeço à minha orientadora Sthefane D' ávila, que acompanhou meu percurso ao longo dos últimos anos e ofereceu uma orientação repleta de conhecimento, sabedoria e paciência. -

Slug: an Emerging Menace in Agriculture: a Review
Journal of Entomology and Zoology Studies 2020; 8(4): 01-06 E-ISSN: 2320-7078 P-ISSN: 2349-6800 www.entomoljournal.com Slug: An emerging menace in agriculture: A JEZS 2020; 8(4): 01-06 © 2020 JEZS review Received: 01-05-2020 Accepted: 03-06-2020 Partha Pratim Gyanudoy Das, Badal Bhattacharyya, Sudhansu Partha Pratim Gyanudoy Das All India Network Project on Bhagawati, Elangbam Bidyarani Devi, Nang Sena Manpoong and K Soil Arthropod Pests, Sindhura Bhairavi Department of Entomology, Assam Agricultural University, Jorhat, Assam, India Abstract Most of the terrestrial slugs are potential threat to agriculture across the globe. Their highly adaptive Badal Bhattacharyya nature helps them to survive in both temperate and tropical climates which is one of the major reasons of All India Network Project on its abundant species diversity. It is not only a severe problem in different seedlings of nursery and Soil Arthropod Pests, orchards, also a worry factor for the seeds of legumes sown in furrows. The whitish slimy mucus Department of Entomology, generated by this pest makes the flower and vegetables unfit for sale. However, despite of its euryphagic Assam Agricultural University, nature, very few works have been carried out on slug morphology, biology, ecology, taxonomy and its Jorhat, Assam, India management in India. This review article tries to integrate the information of economically important slug species of the world as well as India, their bio-ecology, nature of damage, favorable factors with Sudhansu Bhagawati special emphasis on eco-friendly management tactics of this particular gastropod pest. All India Network Project on Soil Arthropod Pests, Keywords: Slug, euryphagic, bio-ecology, management, gastropod pest Department of Entomology, Assam Agricultural University, Jorhat, Assam, India Introduction With a number of 80,000 to 135,000 members, mollusc ranks second largest invertebrate Elangbam Bidyarani Devi group in the world, out of which 1129 species of terrestrial molluscs are found in India [1, 2, 3]. -

Gastropoda: Stylommatophora)1 John L
EENY-494 Terrestrial Slugs of Florida (Gastropoda: Stylommatophora)1 John L. Capinera2 Introduction Florida has only a few terrestrial slug species that are native (indigenous), but some non-native (nonindigenous) species have successfully established here. Many interceptions of slugs are made by quarantine inspectors (Robinson 1999), including species not yet found in the United States or restricted to areas of North America other than Florida. In addition to the many potential invasive slugs originating in temperate climates such as Europe, the traditional source of invasive molluscs for the US, Florida is also quite susceptible to invasion by slugs from warmer climates. Indeed, most of the invaders that have established here are warm-weather or tropical species. Following is a discus- sion of the situation in Florida, including problems with Figure 1. Lateral view of slug showing the breathing pore (pneumostome) open. When closed, the pore can be difficult to locate. slug identification and taxonomy, as well as the behavior, Note that there are two pairs of tentacles, with the larger, upper pair ecology, and management of slugs. bearing visual organs. Credits: Lyle J. Buss, UF/IFAS Biology as nocturnal activity and dwelling mostly in sheltered Slugs are snails without a visible shell (some have an environments. Slugs also reduce water loss by opening their internal shell and a few have a greatly reduced external breathing pore (pneumostome) only periodically instead of shell). The slug life-form (with a reduced or invisible shell) having it open continuously. Slugs produce mucus (slime), has evolved a number of times in different snail families, which allows them to adhere to the substrate and provides but this shell-free body form has imparted similar behavior some protection against abrasion, but some mucus also and physiology in all species of slugs. -

Susceptibility of Biomphalaria Glabrata Submitted to Concomitant Infection with Angiostrongylus Costaricensis and Schistosoma Mansoni L
http://dx.doi.org/10.1590/1519-6984.15215 Original Article Susceptibility of Biomphalaria glabrata submitted to concomitant infection with Angiostrongylus costaricensis and Schistosoma mansoni L. R. Guerinoa, J. F. Carvalhob, L. A. Magalhãesa and E. M. Zanotti-Magalhãesa* aDepartment of Animal Biology, Institute of Biology, State University of Campinas – UNICAMP, Cidade Universitária, Barão Geraldo, CP 6109, CEP 13083-970, Campinas, SP, Brazil bStatistika Consultoria, Rua Barão de Jaguara, 1481, Conjunto 147, CEP 13015-002, Campinas, SP, Brazil *e-mail: [email protected] Received: September 30, 2015 – Accepted: May 3, 2016 – Distributed: August 31, 2017 (With 2 figures) Abstract The easy adaptation of Angiostrongylus costaricensis, nematode responsible for abdominal angiostrongyliasis to several species of terrestrial and freshwater molluscs and the differences observed in the interactions of trematodes with their intermediate hosts have induced us to study the concomitant infection of Biomphalaria glabrata with Schistosoma mansoni and A. costaricensis. Prior exposure of B. glabrata to A. costaricensis (with an interval of 48 hours), favored the development of S. mansoni, observing higher infection rate, increased release of cercariae and increased survival of molluscs, when compared to molluscs exposed only to S. mansoni. Prior exposure of B. glabrata to A. costaricensis and then to S. mansoni also enabled the development of A. costaricensis since in the ninth week of infection, higher amount of A. costaricensis L3 larvae was recovered (12 larvae / mollusc) while for molluscs exposed only to A. costaricensis, the number of larvae recovered was lower (8 larvae / mollusc). However, pre-exposure of B. glabrata to S. mansoni (with an interval of 24 hours), and subsequently exposure to A. -
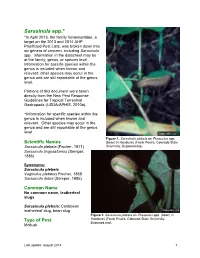
Sarasinula Spp.*
Sarasinula spp.* *In April 2013, the family Veronicellidae, a target on the 2013 and 2014 AHP Prioritized Pest Lists, was broken down into six genera of concern, including Sarasinula spp. Information in the datasheet may be at the family, genus, or species level. Information for specific species within the genus is included when known and relevant; other species may occur in the genus and are still reportable at the genus level. Portions of this document were taken directly from the New Pest Response Guidelines for Tropical Terrestrial Gastropods (USDA-APHIS, 2010a). *Information for specific species within the genus is included when known and relevant. Other species may occur in the genus and are still reportable at the genus level. Figure 1. Sarasinula plebeia on Phaseolus spp. Scientific Names (bean) in Honduras (Frank Peairs, Colorado State Sarasinula plebeia (Fischer, 1871) University, Bugwood.org). Sarasinula linguaeformis (Semper, 1885) Synonyms: Sarasinula plebeia Vaginulus plebeius Fischer, 1868 Sarasinula dubia (Semper, 1885) Common Name No common name, leatherleaf slugs Sarasinula plebeia: Caribbean leatherleaf slug, bean slug Figure 2. Sarasinula plebeia on Phaseolus spp. (bean) in Honduras (Frank Peairs, Colorado State University, Type of Pest Bugwood.org). Mollusk Last update: August 2014 1 Taxonomic Position Class: Gastropoda, Order: Systellommatophora, Family: Veronicellidae Reason for Inclusion in Manual CAPS Target: AHP Prioritized Pest List for FY 2011 – 2015* *Originally listed under the family Veronicellidae. Pest Description Veronicellidae are anatomically distinct from many other terrestrial slugs in that they have a posterior anus, eyes on contractile tentacles, and no pulmonate lung. The sensory tentacles are bilobed. This family also lacks a mantel cavity (Runham and Hunter, 1970). -

Proceedings of the Biological Society of Washington 110(4):520-536
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 110(4):520-536. 1997. Annotated list of Veronicellidae from the collections of the Academy of Natural Sciences of Philadelphia and the National Museum of Natural History, Smithsonian Institution, Washington, D.C., U.S.A. (Mollusca: Gastropoda: Soleolifera) Jose W. Thome, Patricia H. dos Santos, and Luciana Pedott Laboratories de Malacologia, Instituto de Biociencias, PUCRS, Av. Ipiranga, 6681, predio 12; 90619-900 Porto Alegre, RS-Brazil. Abstract.—The list of veronicellid slugs presented in this paper is restricted to species identified by criteria previously proposed by the first author. We were able to distinguish 30 species or subspecies, classified in 12 genera. In- cluded are the following: Belocaulus angustipes; Colosius propinquus; C. pulcher; Diplosolenodes occidentalism D. olivaceus; Heterovaginina peruviana; Laevicaulis alte (with new illustrations); L. natalensis brauni; L. stuhlmanni (with new illustrations); Latipes cnidicaulus; Leidyula dissimilis; L. floridana (with new data and illustration); L. goodfriendi; L. kraussi; L. moreleti; L. portoricensis; L. trichroma; Phyllocaulis gayi; P. soleiformis; Sarasinula du- bia; S. linguaeformis; S. plebeia; Simrothula columbiana; S. prismatica; Va- ginulus taunaisii; Veronicella bahamensis; V. cubensis; V. davisi; V. sloanei; V. tenax. The family Veronicellidae Gray, 1840 groups of characteristics to be analyzed. He comprises terrestrial mollusks without shell, comments on the characteristics tradition- commonly called slugs. The distribution is ally used and is of the opinion that there are pantropical and over 300 specific names not enough to determine conclusively the have been registered, most of them syn- species, and that they do not permit a con- onyms (Hoffmann 1925, Forcart 1953, sistent phylogenetic classification. -

Cynthia De Paula Andrade
Universidade Federal de Minas Gerais Instituto de Ciências Biológicas Programa de Pós-Graduação em Parasitologia Angiostrongylus vasorum (Nematoda: Protostrongylidae): Aspectos do desenvolvimento dos estádios evolutivos em Melanoides tuberculata (Caenogastropoda: Thiaridae), Bradybaena similaris (Stylommatophora: Xanthonychidae) e Sarasinula marginata (Soleolifera: Veronicellidae) Cynthia de Paula Andrade Belo Horizonte - MG 2012 Cynthia de Paula Andrade Angiostrongylus vasorum (Nematoda, Protostrongylidae): Aspectos do desenvolvimento dos estádios evolutivos em Melanoides tuberculata (Caenogastropoda: Thiaridae), Bradybaena similaris (Stylommatophora: Xanthonychidae) e Sarasinula marginata (Soleolifera: Veronicellidae) Dissertação apresentada ao Departamento de Parasitologia do Instituto de Ciências Biológicas da Universidade Federal de Minas Gerais, como parte dos requisitos necessários para a obtenção do grau de Mestre em Parasitologia Área de Concentração: Helmintologia Orientadora: Profª. Drª. Teofânia Heloísa Dutra Amorim Vidigal1 Co-orientador: Prof. Dr. Walter dos Santos Lima2 Colaboradores: Lanuze Rose Mozzer Soares2, Lângia Colli Montresor3 1- Laboratório de Malacologia e Sistemática Molecular, Depto. Zoologia, UFMG 2- Laboratório de Helmintologia Veterinária, Depto. Parasitologia, UFMG, 3- Laboratório de Malacologia, Instituto Oswaldo Cruz - IOC, Fiocruz, RJ. Belo Horizonte – MG 2012 Digo: o real não está na saída nem na chegada: ele se dispõe para a gente é no meio da travessia. Guimarães Rosa AGRADECIMENTOS Agradeço especialmente -

Snail and Slug Dissection Tutorial: Many Terrestrial Gastropods Cannot Be
IDENTIFICATION OF AGRICULTURALLY IMPORTANT MOLLUSCS TO THE U.S. AND OBSERVATIONS ON SELECT FLORIDA SPECIES By JODI WHITE-MCLEAN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2012 1 © 2012 Jodi White-McLean 2 To my wonderful husband Steve whose love and support helped me to complete this work. I also dedicate this work to my beautiful daughter Sidni who remains the sunshine in my life. 3 ACKNOWLEDGMENTS I would like to express my sincere gratitude to my committee chairman, Dr. John Capinera for his endless support and guidance. His invaluable effort to encourage critical thinking is greatly appreciated. I would also like to thank my supervisory committee (Dr. Amanda Hodges, Dr. Catharine Mannion, Dr. Gustav Paulay and John Slapcinsky) for their guidance in completing this work. I would like to thank Terrence Walters, Matthew Trice and Amanda Redford form the United States Department of Agriculture - Animal and Plant Health Inspection Service - Plant Protection and Quarantine (USDA-APHIS-PPQ) for providing me with financial and technical assistance. This degree would not have been possible without their help. I also would like to thank John Slapcinsky and the staff as the Florida Museum of Natural History for making their collections and services available and accessible. I also would like to thank Dr. Jennifer Gillett-Kaufman for her assistance in the collection of the fungi used in this dissertation. I am truly grateful for the time that both Dr. Gillett-Kaufman and Dr. -

ZM82 615-650 Robinson.Indd
The land Mollusca of Dominica (Lesser Antilles), with notes on some enigmatic or rare species D.G. Robinson, A. Hovestadt, A. Fields & A.S.H. Breure Robinson, D.G., A. Hovestadt, A. Fields & A.S.H. Breure. The land Mollusca of Dominica, Lesser Antilles, with notes on some enigmatic or rare species. Zool. Med. Leiden 83 (13), 9.vii.2009: 615-650, fi gs 1-14, tables 1-2.― ISSN 0024-0672. D.G. Robinson, The Academy of Natural Sciences, 1900 Benjamin Franklin Parkway, Philadelphia, PA 19103, U.S.A. ([email protected]). A. Hovestadt, Dr. Abraham Kuyperlaan 22, NL-3818 JC Amersfoort, The Netherlands (ad.hovestadt@ xs4all.nl). A. Fields, Department of Biological and Chemical Sciences, University of the West Indies, Cave Hill Cam- pus, Barbados (angela.fi [email protected]). A.S.H. Breure, National Museum of Natural History, P.O. Box 9517, NL-2300 RA Leiden, The Netherlands ([email protected]). Key words: Mollusca, Gastropoda, taxonomy, distribution, Dominica. An overview of the land-snail fauna of the Lesser Antillean island of Dominica is given, based on data from literature and four recent surveys. There are 42 taxa listed, of which the following species are recorded for the fi rst time from the island: Allopeas gracile (Hutt on, 1834), A. micra (d’Orbigny, 1835), Beckianum beckianum (L. Pfeiff er, 1846), Bulimulus diaphanus fraterculus (Potiez & Michaud, 1835), De- roceras laeve (Müller, 1774), Sarasinula marginata (Semper, 1885), Streptostele musaecola (Morelet, 1860) and Veronicella sloanii (Cuvier, 1817). The enigmatic Bulimulus stenogyroides Guppy, 1868 is now placed in the genus Naesiotus Albers, 1850. -

Species List for Garey Park-Inverts
Species List for Garey Park-Inverts Category Order Family Scientific Name Common Name Abundance Category Order Family Scientific Name Common Name Abundance Arachnid Araneae Agelenidae Funnel Weaver Common Arachnid Araneae Thomisidae Misumena vatia Goldenrod Crab Spider Common Arachnid Araneae Araneidae Araneus miniatus Black-Spotted Orbweaver Rare Arachnid Araneae Thomisidae Misumessus oblongus American Green Crab Spider Common Arachnid Araneae Araneidae Argiope aurantia Yellow Garden Spider Common Arachnid Araneae Uloboridae Uloborus glomosus Featherlegged Orbweaver Uncommon Arachnid Araneae Araneidae Argiope trifasciata Banded Garden Spider Uncommon Arachnid Endeostigmata Eriophyidae Aceria theospyri Persimmon Leaf Blister Gall Rare Arachnid Araneae Araneidae Gasteracantha cancriformis Spinybacked Orbweaver Common Arachnid Endeostigmata Eriophyidae Aculops rhois Poison Ivy Leaf Mite Common Arachnid Araneae Araneidae Gea heptagon Heptagonal Orbweaver Rare Arachnid Ixodida Ixodidae Amblyomma americanum Lone Star Tick Rare Arachnid Araneae Araneidae Larinioides cornutus Furrow Orbweaver Common Arachnid Ixodida Ixodidae Dermacentor variabilis American Dog Tick Common Arachnid Araneae Araneidae Mangora gibberosa Lined Orbweaver Uncommon Arachnid Opiliones Sclerosomatidae Leiobunum vittatum Eastern Harvestman Uncommon Arachnid Araneae Araneidae Mangora placida Tuft-legged Orbweaver Uncommon Arachnid Trombidiformes Anystidae Whirligig Mite Rare Arachnid Araneae Araneidae Mecynogea lemniscata Basilica Orbweaver Rare Arachnid Eumesosoma roeweri -

Infecção Natural Por Larvas De Metastrongilídeos Em Moluscos Terrestres De Diferentes Regiões Do Estado De São Paulo
DAN JESSÉ GONÇALVES DA MOTA Infecção natural por larvas de metastrongilídeos em moluscos terrestres de diferentes regiões do estado de São Paulo Tese apresentada ao Programa de Pós- Graduação em Ciências da Coordenadoria de Controle de Doenças da Secretaria de Estado da Saúde de São Paulo, para obtenção do título de Doutor em Ciências. Área de concentração: Pesquisas Laboratoriais em Saúde Pública Orientador: Prof. Dr. Pedro Luiz Silva Pinto SÃO PAULO 2018 ’’Os mais poderosos intelectos da Terra não podem compreender a Deus. Os homens podem estar sempre a pesquisar, sempre a aprender, e ainda há, para além, um infinito de conhecimento’’. Ellen G. White DEDICATÓRIA Aos meus pais, Ira e Lauro. Aos meus irmãos Adriana, Damaris, Jonas e Tamine. A minha esposa Valéria. Aos meus sobrinhos, Ádila, Júlia, Bruno e Sofia. Aos cunhados Robson e Felipe. Obrigado por vocês terem sido em minha vida modelos reais de amor, amizade, apoio, carinho e compreensão. AGRADECIMENTOS Agradeço ao Prof. Dr.Pedro Luiz Silva Pinto, pela oportunidade e confiança em mim depositada ao longo desses anos, pela orientação, incentivo e importante participação na minha formação científica. Ao Programa de Pós - Graduação em Ciências da Coordenadoria de Controle de Doenças da Secretaria de Estado da Saúde de São Paulo pela oportunidade de realização do doutorado. Minha gratidão a todos docentes e colegas de curso que contribuíram com minha formação acadêmica. Ao Núcleo de Enteroparasitas do Instituto Adolfo Lutz pelo apoio e acolhimento muito importantes nesta etapa de minha formação, agradeço a todos os funcionários que de várias maneiras apoiaram a execução deste trabalho. -

Medical and Veterinary Aspects of Snails and Slugs
Snails and slugs aren’t just agricultural and horticultural pests David G. Robinson Ph.D. USDA APHIS National Malacology Laboratory The Academy of Natural Sciences of Drexel University, Philadelphia, PA 19103 USA Invasive mollusk species have the potential to: Introduce snail-vectored human and livestock diseases, including cerebral and abdominal angiostrongyliasis Introduce these diseases to native species and other invasive species that are already present Laboratory studies indicate that diseases such as those that affect recognized vectors (such as GAS and veronicellid slugs) can be introduced into native snail and slug populations, increasing the threat to Public Health Facilitate the spread of other diseases not normally associated with mollusks For example, large accumulations of dead GAS shells filling with rain water can provide an ideal breeding environment for mosquito larvae – producing more mosquitoes and perhaps facilitating the spread of malaria, dengue fever, yellow fever and as of 2015 chikungunya (throughout the West Indies, including the U.S. Virgin Islands), as well as Africa (where it originated) Plant pathogens such as Phytophtora spp. , not usually associated with molluscs, can also be vectored by snails including GAS Dead shells along roadside in Barbados (photo: A. Fields) The association of invasive, synanthropic snails and slugs with human habitation brings snails and slugs, if populations are high enough, into direct contact with people Directly impact urban and suburban householders, leaving slime trails, piles of feces and dead, rotting snails in homes and gardens This may also impact tourism Example: GAS on golf courses, in hotels in hotel gardens, floating dead in swimming pools, etc.