Geochemistry of a Paleosol Horizon at the Base of the Sausar Group, Central India: Implications on Atmospheric Conditions at the Archeanepaleoproterozoic Boundary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of 6038 Schools Selected for Establishment of Atal Tinkering
LIST OF 6038 SCHOOLS SELECTED FOR ESTABLISHMENT OF ATAL TINKERING LABS (SCHOOLS ARE KINDLY REQUESTED TO WAIT FOR FURTHER INSTRUCTIONS FROM ATAL INNOVATION MISSION, NITI AAYOG ON THEIR REGISTERED EMAIL IDs) PLEASE NOTE:- 1. LAST DATE FOR COMPLETING THE COMPLIANCE PROCESS : 31st JANUARY 2020 2. THESE SELECTED SCHOOLS MUST OPEN A NEW BANK ACCOUNT IN A PUBLIC SECTOR BANK FOR THE PURPOSE OF ATL GRANT. 3. THESE SELECTED SCHOOLS MUST NOT SHARE THEIR INFORMATION WITH ANY THIRD PARTY/ VENDOR/ AGENT/ AND MUST COMPLETE THE COMPLIANCE PROCESS ON THEIR OWN. 4. THIS LIST IS ARRANGED IN ALPHABETICAL ORDER OF STATE, DISTRICT AND FINALLY SCHOOL NAME. S.N. ATL UID CODE UDISE CODE SCHOOL NAME STATE DISTRICT 1 2760806 28222800515 ANDHRA PRADESH MODEL SCHOOL PUTLURU ANDHRA PRADESH ANANTAPUR 2 132314217 28224201013 AP MODEL SCHOOL ANDHRA PRADESH ANANTAPUR 3 574614473 28223600320 AP MODEL SCHOOL AND JUNIOR COLLEGE ANDHRA PRADESH ANANTAPUR 4 278814373 28223200124 AP MODEL SCHOOL RAPTHADU ANDHRA PRADESH ANANTAPUR 5 2995459 28222500704 AP SOCIAL WELFARE RESIDENTIAL SCHOOL JUNIOR COLLEGE FOR GIRLS KURUGUNTA ANDHRA PRADESH ANANTAPUR 6 13701194 28220601919 AVR EM HIGH SCHOOL ANDHRA PRADESH ANANTAPUR 7 15712075 28221890982 AVR EM HIGH SCHOOL ANDHRA PRADESH ANANTAPUR 8 56051196 28222301035 AVR EM HIGH SCHOOL ANDHRA PRADESH ANANTAPUR 9 385c1160 28221591153 AVR EM HIGH SCHOOL ANDHRA PRADESH ANANTAPUR 10 102112978 28220902023 GOOD SHEPHERD ENGLISH MEDIUM SCHOOL ANDHRA PRADESH ANANTAPUR 11 243715046 28220590484 K C NARAYANA E M SCHOOL ANDHRA PRADESH ANANTAPUR LIST OF 6038 SCHOOLS SELECTED FOR ESTABLISHMENT OF ATAL TINKERING LABS (SCHOOLS ARE KINDLY REQUESTED TO WAIT FOR FURTHER INSTRUCTIONS FROM ATAL INNOVATION MISSION, NITI AAYOG ON THEIR REGISTERED EMAIL IDs) PLEASE NOTE:- 1. -

Annual Report 2014-15.Pdf
YASHADA YASHWANTRAO CHAVAN ACADEMY OF DEVELOPMENT ADMINISTRATION The Yashwantrao Chavan Academy of Prime Minister of India, Shri Yashwantrao B. Development Administration (YASHADA) was Chavan. In 1884, it shifted its location to Pune, and established by the Government of Maharashtra to was named the Maharashtra Institute of impart training to government officials and elected Development Administration or MIDA. Its main representatives, conduct research and suggest policy objective was to serve as the apex body in the recommendations. promotion and development of modern management science, and function as the nodal state level training Maharashtra was one of the first states in the institute in the field of development administration. country to realise the importance of human resources development. The early sixties witnessed After six successful years, on the 26th of November, the establishment of the Administrative Staff 1990, MIDA graduated into an Academy with a new College (ASC) in Mumbai, under the inspiration of name, the Yashwantrao Chavan Academy of former Chief Minister of Maharashtra and Deputy Development Administration…. YASHADA. 1 Objectives Types of Activities: The objectives of the Academy as listed in its • Memorandum of Association (MoA) are Training programmes of short and long term duration including seminars, workshops, and conferences, as a means of continuing education • To promote modern management science as a for practicing managers, administrators and major instrument for development of economic scientific and technical cadres; and social activities of the State Government, Zilla Parishads and other institutions and • Policy oriented & operational research; organisations of the State Government. • Consultancy and extension services; & • To develop managerial skills, organisational • capability, leadership and decision-making Publication & production of training aids. -
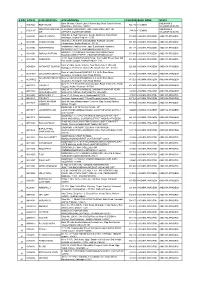
S No Atm Id Atm Location Atm Address Pincode Bank
S NO ATM ID ATM LOCATION ATM ADDRESS PINCODE BANK ZONE STATE Bank Of India, Church Lane, Phoenix Bay, Near Carmel School, ANDAMAN & ACE9022 PORT BLAIR 744 101 CHENNAI 1 Ward No.6, Port Blair - 744101 NICOBAR ISLANDS DOLYGUNJ,PORTBL ATR ROAD, PHARGOAN, DOLYGUNJ POST,OPP TO ANDAMAN & CCE8137 744103 CHENNAI 2 AIR AIRPORT, SOUTH ANDAMAN NICOBAR ISLANDS Shop No :2, Near Sai Xerox, Beside Medinova, Rajiv Road, AAX8001 ANANTHAPURA 515 001 ANDHRA PRADESH ANDHRA PRADESH 3 Anathapur, Andhra Pradesh - 5155 Shop No 2, Ammanna Setty Building, Kothavur Junction, ACV8001 CHODAVARAM 531 036 ANDHRA PRADESH ANDHRA PRADESH 4 Chodavaram, Andhra Pradesh - 53136 kiranashop 5 road junction ,opp. Sudarshana mandiram, ACV8002 NARSIPATNAM 531 116 ANDHRA PRADESH ANDHRA PRADESH 5 Narsipatnam 531116 visakhapatnam (dist)-531116 DO.NO 11-183,GOPALA PATNAM, MAIN ROAD NEAR ACV8003 GOPALA PATNAM 530 047 ANDHRA PRADESH ANDHRA PRADESH 6 NOOKALAMMA TEMPLE, VISAKHAPATNAM-530047 4-493, Near Bharat Petroliam Pump, Koti Reddy Street, Near Old ACY8001 CUDDAPPA 516 001 ANDHRA PRADESH ANDHRA PRADESH 7 Bus stand Cudappa, Andhra Pradesh- 5161 Bank of India, Guntur Branch, Door No.5-25-521, Main Rd, AGN9001 KOTHAPET GUNTUR 522 001 ANDHRA PRADESH ANDHRA PRADESH Kothapeta, P.B.No.66, Guntur (P), Dist.Guntur, AP - 522001. 8 Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW8001 GAJUWAKA BRANCH 530 026 ANDHRA PRADESH ANDHRA PRADESH 9 Gajuwaka, Anakapalle Main Road-530026 GAJUWAKA BRANCH Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW9002 530 026 ANDHRA PRADESH ANDHRA PRADESH -

From Vidarbha Region (MS) India
Bioscience Discovery, 5(2):180-186, July - 2014 © RUT Printer and Publisher (http://jbsd.in) ISSN: 2229-3469 (Print); ISSN: 2231-024X (Online) Received: 14-06-2014, Revised: 25-06-2014, Accepted: 27-06-2014e Full Length Article Use of Ethno-Veterinary medicines (EVM) from Vidarbha Region (MS) India Sajal Kulkarni, DK Kulkarni, AD Deo, AB Pande and RL Bhagat BAIF Development Research Foundation, Pune 411058. ABSTRACT Rural tribal people generally depend on plants for curing their livestock due to inadequate facilities provided by Government agencies. The field survey was carried out in 3 clusters of Nagpur, Chandrapur and Gadchiroli of Maharashtra State. Local people were using 46 plants to cure certain veterinary diseases commonly occurring in region. Sixty people are experienced in giving plant based herbal drugs and the doses are very effective in treatments. The present study deals with different ethno-veterinary practices performed by local farmers and Vaidus to treat their livestock.The enumerations of different veterinary diseases and their plant resources are listed along with their parts used, local use and local name from study area. Key words: EVM, livestock diseases, Vaidus, Chandrapur, Nagpur and Gadchiroli. INTRODUCTION diseases, overwork and old age (Shammasastry, India has ancient roots of utilizing plant 2004). The cow herd’s people have the knowledge resources for curing diseases of horses and cow of many animal diseases from which cattle might herds. In the ancient India, Rigveda is one of the suffer and also knew the plant based remedies like old literature mentioning cattle and their garlic (Allum sativum L.) Pipali Mula (Piper longum management. -

District Census Handbook, Nagpur
·CENSUS OF INDIA 1961 DISTRICT CENSUS HANDBOOK NAGPUR Compiled by THE MAHARASHTRA CENSUS OFFICE BOMBAY Printed in India by the Manager, Government Press and Book Depot, Nagpur, and Published by the Director, Government Printing and Stationery, Maharashtra State, Bombay-,. 1965 rPrice - Rs. Ei~ht] CENSUS OF INDIA 196 t Central Government Publications Census Report, Volume X-Maharashtra, is published in the folk>wing Parts I-A and B General Report I-C Subsidiary Tables II-A General Popuiation Tables JI-B (i) General Economic Tables-Industrial Classification II-]} (ii) General Economic Tables-Occupational Classification II-C (i) Social and Cultural Tables II-C (ii) Migration Tables III Household Economic Tables IV Report on Housing and Establishments V-A Scheduled Castes and Scheduled Tribes in Maharashtra-Tables V·B ... Scheduled Castes and Scheduled Tribes i.n Maharashtra-Ethnographic Notes VI (1-35) Village Surveys (35 monographs on 35 selected villages) VII·A Handicrafts in Maharashtra VII-B Fairs and Festivals in Maharashtra VIII-A Administration Report-Enumeration (For official use only) VIII·B Administration Report-Tabulation (For official use only) IX Census Atlas of Maharashtra X (1-12) Cities of Maharashtra (15 Volumes-Four volumes on Greater Bombay and One each on other eleven Cities) State Government Publications 25 Volumes of District Census Handbooks in English 25 Volumes of District Census Handbooks in Marathi Alphabetical List of Villages in Maharashtra J-1977-i.3-(Nagpur.} ' . .. : « Drs ~ A r I? I l (r.. 0 D l c .. O c t- % I ~ " 0 III , It! 0 0 l- I- " ' ~ \ c:r . '" .(, ... ~'" ,..~ • A,lIAr q. -

Vocational Survey Report Nagpur District
VOCATIONAL SURVEY REPORT NAGPUR DISTRICT N IE P A D C Mb. N«fioiml Systran National Institute yf EdiaofttiolMd Ptenmnf and ’ mm « « *•, INDEX Chapter No. Description Page No, Preface i to iii t I) Objective and Methodology for J -j-o II conducting Vocational study/ survey. II) District Profiite 32. to SI Ill) Industrial growth and employment retrospect. 82 to 122. IV) Vocational education 123 to ) S>*7 current status and study in Futurology. S SUMMARY* f % % / 9 / i) Summary of Vocational Courses __ £c LJJ. identified. j Q ^_ ii )Tahasilwise summaiy cf ___ £0 Vocational courses identified. APPENDJCBS / 1 .) Land Use Pa tern, 1985-86 203 to 206 2.) Tahasilwise classification of villages according to ^0/ to 208. population ranges. 3) 7'ahasilv/ise ocupat ional pa tern 209 to 210r 4) TahasiIvise area under xxBpsx 211 to 2.18 different crops. 5) Tahasilwise area undei irrigated ciops. 219 to 222 6) Live Stock Information 223 to 228. 7)Agricultural Narked Commitee. 229 top 231, < i ) P RE F ft £ E Education has important role to play in the process of economic development* Its main function is to create human capital by augmenting the supply of trained, competent manpower for economic development, The Education is a unique investment in the present a n d f u t u r e * T h e g r o w t h o f e in p 1 o y m e n t o p p o r t u n i t i e s a ri d h u m a n resourc e deve 1 opment must be inter--dependant for ba 1 anc ed deve 1 op•••■ ment of economy* The Education which develops specific skill relevant to economy and environment should receive -

Nagpur District at a Glance 1
NAGPUR DISTRICT AT A GLANCE 1. GENERAL INFORMATION Geographical Area : 9892 sq. km. Administrative Divisions : Taluka-14; Nagpur(Urban), Kamptee, (As on 31/03/2011) Mouda, Ramtek, Parseoni, Saoner, Kalmeshwar, Narkhed, Katol, Hingna, Nagpur(Rural), Umred, Kuhi, Bhiwapur. Villages : 1562 Population (2001 Census) : 40.51 Lakh Normal Annual Rainfall : 1000 mm to 1200 mm 2. GEOMORPHOLOGY Major Physiographic unit : Three; Northern hilly area, Western hilly area and Kanhan & Wena river valley area Major Drainage : Two; Wardha and Wainganga 3. LAND USE (2000-01) Forest Area : 1350 sq. km. Net Area Sown : 5074 sq. km. Cultivable Area : 5759 sq. km. 4. SOIL TYPE Medium to deep clayey, black cotton soils; medium, loamy alluvial soils; shallow sandy, clayey red soils. 5. PRINCIPAL CROPS (2010-11) Jowar : 525 sq. km. Cotton : 374 sq. km. Wheat : 310 sq. km. Rice : 434 Sq km Total Pulses : 936 sq. km. Soyabean 2069 sq km Fruits : 343 sq km 6. IRRIGATION BY DIFFERENT SOURCES (2006-7) Nos./Potential Created (ha) Dugwells : 69695 / 140154 Borewells and Tubewells : 127 / 246 Surface Flow Schemes : 732/1278 Surface Kift Schemes : 14589 / 41576 Net Irrigated Area : 183254 ha 7. GROUND WATER MONITORING WELLS (As on 31/03/2012) Dugwells : 43 Piezometers : 18 8. GEOLOGY Recent : Alluvium Upper Cretaceous-Lower Eocene : Deccan Trap Basalt Cretaceous : Lameta Permian to Carboniferous : Gondwana Archean : Crystalline rocks 9. HYDROGEOLOGY Water Bearing Formation : Archean- Weathered and fractured Granite Gneiss, Gondwana- Kamptee and Barakar Sandstone, Trap- covered Gondwana; Deccan Trap-Weathered/Fractured /Jointed Massive or Vesicular Basalt; Alluvium- Sand and Gravel i Premonsoon Depth to Water Level : 0.08 to 15.59 m bgl (May-2011) Postmonsoon Depth to Water Level : 0.60 to 10.60 m bgl (Nov.-2011) Premonsoon Water Level Trend : Rise: 0.02 to 0.54 m/year (2001-2010) Fall: 0.006 to 0.86 m/year Postmonsoon Water Level Trend : Rise: Negligible to 0.63 m/year (1998-2007) Fall: 0.001 to 0.24 m/year 10. -

TMC Submits Memorandum to EC, Urges Holding of Bypolls in Bengal
MILLENNIUMPOST.IN RNI NO.: WBENG/2015/65962 PUBLISHED FROM DELHI | KOLKATA VOL. 7, ISSUE 234 | Friday, 27 August 2021 | Kolkata | Pages 12 | Rs 3.00 No Half Truths City: Pg 2 Nation: Pg 6 Film: Pg 12 ‘Industries will not face delay in Four-day Malabar wargame begins ‘I aim at touching getting nod for projects in state’ off coast of Guam in Western Pacific people’s hearts’ Unitech: SC furious as ED submits Chandras running ‘secret office’ with help from jailers Orders Sanjay and Ajay Chandra’s transfer to Arthur Road and Taloja jails; directs CP, Delhi to personally conduct inquiry OUR CORRESPONDENT Following these materials being raised some serious and disturb- sonally and shall not be delegated to ities by flouting the jail manual, aspects which have been adverted to made available to the top court in ing issues. any other officer. making transfers of assets and dis- in the communication to the Com- NEW DELHI: In a set of startling two separate reports, a Bench of It said that the video conferenc- Significantly, in their reports, the sipating the proceeds of crime and missioner of Police.” revelations in the Supreme Court, Justices DY Chandrachud and MR ing facility at the jails to which the ED has told the top court that they influencing witnesses and attempt- It said that letter’s content and the Enforcement Directorate has Shah on Thursday directed that accused are being transferred shall had unearthed the “secret office” in ing to derail the investigation. It the material which has come on said the Chandra brothers — Sanjay erstwhile Unitech promoters San- be made available so as to enable South Delhi and found that it was added that an ED investigator had record indicate that, despite the and Ajay — of the Unitech Group jay and Ajay Chandra be moved out them to record their presence in the being operated by erstwhile Uni- already written to the Delhi Police orders of this Court, irregularities had been operating a “secret under- of Tihar Jail here and be shifted to court proceedings where their pres- tech founder Ramesh Chandra. -

2020031344.Pdf
DISTRICT SURVEY REPORT (For Sand Mining) (As per Ministry of Environment, Forest and Climate Change, G.O.I, New Delhi; Notification No. S. O. 3611 (E), Dated - 25th July, 2018.) 2020 CHAPTER 1 INTRODUCTION Nagpur is the winter capital of the state of Maharashtra, a fast growing metropolis and third largest city in Maharashtra after Mumbai and Pune. With a population of 2,405,421 (2011) Nagpur Metropolitan Area is the 13th largest urban conglomeration in India. It has also recently been ranked as the cleanest city and the second greenest city of India. In addition to being the seat of annual winter session of Maharashtra state assembly “Vidhan Sabha”, Nagpur is also a major commercial and political center of the Vidharbha region of Maharashtra, and is also famous throughout the country as “Orange City” for being a major trade center of oranges that are cultivated in the region. In addition, the city also derives political importance from being the headquarters for the RSS and an important location for the Buddhistmovement. The city was founded by the Gonds but later became part of the Maratha Empire under the Bhonsles. The British East India Company took over Nagpur in the 19th century and made it the capital of the Central Provinces and Berar. After the first reorganization of states, the city lost its capital status but according to the “Nagpur Pact” between political leaders it was made the second capital of Maharashtra. Pic 1: Central Provinces and Berar, 1903. Nagpur is also called, “Tiger Capital of India” as it connects many Tiger Reserves in India to the world. -

THERIOPHONUM INDICUM (DALZ.) ENGLER. (ARACEAE) –LEAFY VEGETABLE of GONDIA TRIBE, VIDARBHA REGION, MAHARASHTRA Suwarna Deshpande and *Kulkarni D.K
Indian Journal of Fundamental and Applied Life Sciences ISSN: 2231-6345 (Online) An Online International Journal Available at http://www.cibtech.org/jls.htm 2013 Vol. 3 (4) October-December, pp.35-38/Suwarna and Kulkarni Research Article THERIOPHONUM INDICUM (DALZ.) ENGLER. (ARACEAE) –LEAFY VEGETABLE OF GONDIA TRIBE, VIDARBHA REGION, MAHARASHTRA Suwarna Deshpande and *Kulkarni D.K. BAIF Development Research Foundation, Pune- 411 058 *Author for Correspondence ABASTRACT Local vegetables are consumed by Gondia trine from Deolapar Region at Nagpur District, Vidarbha region (Altitude: 1331 feet. N=21°37’21’.948”. E=79° 20’39’.944”). Toxic vegetables like Theriophonum indicum (Dalz) Engler required special preparation method to make it edible. This rare plant available during rainy season and first report of edible use is mentioned in the note. INTRODUCTION Aborigines consume a main staple diet and it is supported with supplementary wild foods. These species are consumed by various communities depending on the local availability. Various preparations of plant species are prepared and sold in tribal markets. Tribals and local communities have accurate knowledge of wild food resources due to their long association with nature. Jain and Sinha (1988) reported food resources from Indian aborigines. These tribals consume 144 non-conventional food resources which are playing major role in nutritional point of view (Kulkarni, 2006). Majority of tribals consume members of family Araceae as vegetables. Some species of Alocasia, tubers of Amorphophallus, leafy vegetables of Ariopsis. Colocasia, Remusatia, flowering stalk of Sauromatum. Most of these plants have some irritating substance and they prepare it very carefully washing and adding some sour plant leaf for removing toxicity during the cooking. -

Chapter – 5 Hydrology and Water Assessment
Chapter – 5 Hydrology and Water Assessment 5.0 General The proposed Wainganga (Gosikhurd) - Nalganga (Purna Tapi) intra-state link project of Maharashtra envisages diversion of 1772 Mm3 from the existing Gosikhurd (Indira Sagar) project (first National Project in the country) on Wainganga river in Pranhita sub-basin of Godavari basin for extending irrigation, domestic and industrial water supply benefits in six districts of Vidarbha region of Maharashtra State viz., Nagpur, Wardha, Amravati, Yeotmal, Akola and Buldhana before out falling into Nalganga project on Nalganga river of Tapi basin. Seasonal climate prevails in the link project area. Four distinct seasons are noticeable in the year viz., Monsoon from June to October, Post-monsoon from October to mid December, Winter from mid December to February and Summer from March to May. The climate is normally cold from December to February which is followed by hot season from March to May. The hydrology and water availability studies of the project are based on the Rainfall-Runoff relationships developed for four sub-catchments within the catchment of Wainganga at Gosikhurd dam site. 5.1 General Information about Regions (a) Wainganga/Pranhita Sub-basin of Godavari Basin The salient features/general information of the four sub-catchments upto Gosikhurd dam site in Wainganga sub-basin viz. Pench, Kanhan, Bagh and Wainganga is described as under: (i) Pench Sub-catchment The Pench is a tributary of the Kanhan River. It originates in the Chhindwara district of Madhya Pradesh and flows for about 200 km in the district and then for 56 km through Nagpur district of Maharashtra before joining Kanhan river. -

Exploration & Development 14-15
EXPLORATION & DEVELOPMENT Indian Minerals Yearbook 2015 (Part- I : GENERAL REVIEWS) 54th Edition EXPLORATION & DEVELOPMENT (ADVANCE RELEASE) GOVERNMENT OF INDIA MINISTRY OF MINES INDIAN BUREAU OF MINES Indira Bhavan, Civil Lines, NAGPUR – 440 001 PHONE/FAX NO. (0712) 2565471 PBX : (0712) 2562649, 2560544, 2560648 E-MAIL : [email protected] Website: www.ibm.gov.in March, 2017 4-1 EXPLORATION & DEVELOPMENT 4 Exploration & Development GOVERNMENT’S POLICY Data filing will be rigorously applied and he National Mineral Policy, 2008 for non- concession holders will be monitored. Tfuel and non-coal minerals, introduced by the Lock-in arrangement will be assured and Government in replacement of the National Mineral the data will be released to prospectors Policy 1993 lay enormous thrust on the various after integration. aspects of mineral industry, such as regulation of * Prospecting being a high-risk venture, minerals, role of State in mineral development, access to risk funds from capital markets survey and exploration, database of mineral will be facilitated. resources and tenements, strategy of mineral development, etc. Among other things, strong This policy initiative is expected to encourage emphasis is laid on the following: greater involvement of private sector in survey and exploration of minerals. * To judiciously exploit and utilise the country's mineral potentialities, The High-Level Committee constituted by the systematic regional and detailed Government of India which brought out the exploration will be carried out using state- National Mineral Policy, 2008 has recommended of-the-art techniques in a time bound amendments to the MMDR Act, 1957 with the manner. Zero-waste mining will be the purpose of providing necessary initiatives to national goal and mining technology will attract investment and participation of private and be upgraded to ensure exploration and public sectors in areas of exploration and utilisation of entire run-of-the-mine.