Transposons – the Useful Genetic Tools
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transposon Mutagenesis in Streptomycetes
Transposon Mutagenesis in Streptomycetes Dissertation zur Erlangung des Grades des Doktors der Naturwissenschaften der Naturwissenschaftlich-Technischen Fakultät III Chemie, Pharmazie, Bio- und Werkstoffwissenschaften der Universität des Saarlandes von Bohdan Bilyk Saarbrücken 2014 Tag des Kolloquiums: 6. Oktober 2014 Dekan: Prof. Dr. Volkhard Helms Berichterstatter: Dr. Andriy Luzhetskyy Prof. Dr. Rolf Müller Vorsitz: Prof. Dr. Claus-Michael Lehr Akad. Mitarbeiter: Dr. Mostafa Hamed To Danylo and Oksana IV PUBLICATIONS Bilyk, B., Weber, S., Myronovskyi, M., Bilyk, O., Petzke, L., Luzhetskyy, A. (2013). In vivo random mutagenesis of streptomycetes using mariner-based transposon Himar1. Appl Microbiol Biotechnol. 2013 Jan; 97(1):351-9. Bilyk, B., Luzhetskyy, A. (2014). Unusual site-specific DNA integration into the highly active pseudo-attB of the Streptomyces albus J1074 genome. Appl Microbiol Biotechnol. Accepted CONFERENCE CONTRIBUTIONS Bilyk, B., Weber, S., Myronovskyi, M., Luzhetskyy, A. Himar1 in vivo transposon mutagenesis of Streptomyces coelicolor and Streptomyces albus. Poster presentation at International VAAM Workshop, University of Braunschweig, September 27-29, 2012. Bilyk, B., Weber, S., Welle, E., Luzhetskyy, A. Himar1 in vivo transposon mutagenesis of Streptomyces coelicolor. Poster presentation at International VAAM Workshop, University of Bonn, September 28 – 30, 2011. Bilyk, B., Weber, S., Welle, E., Luzhetskyy, A. In vivo transposon mutagenesis of streptomycetes using a modified version of Himar1. Poster presentation at International VAAM Workshop, University of Tübingen, September 26 -28, 2010. V TABLE OF CONTENTS SUMMARY XIII 1. INTRODUCTION 15 1.1. Streptomycetes, organisms with outstanding potential 15 1.1.1. Phylogeny of actinomycetes 15 1.1.2. Streptomyces 15 1.1.3. Exploiting the potential of streptomycetes as antibiotic producers. -

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Horizontal Gene Transfer
Genetic Variation: The genetic substrate for natural selection Horizontal Gene Transfer Dr. Carol E. Lee, University of Wisconsin Copyright ©2020; Do not upload without permission What about organisms that do not have sexual reproduction? In prokaryotes: Horizontal gene transfer (HGT): Also termed Lateral Gene Transfer - the lateral transmission of genes between individual cells, either directly or indirectly. Could include transformation, transduction, and conjugation. This transfer of genes between organisms occurs in a manner distinct from the vertical transmission of genes from parent to offspring via sexual reproduction. These mechanisms not only generate new gene assortments, they also help move genes throughout populations and from species to species. HGT has been shown to be an important factor in the evolution of many organisms. From some basic background on prokaryotic genome architecture Smaller Population Size • Differences in genome architecture (noncoding, nonfunctional) (regulatory sequence) (transcribed sequence) General Principles • Most conserved feature of Prokaryotes is the operon • Gene Order: Prokaryotic gene order is not conserved (aside from order within the operon), whereas in Eukaryotes gene order tends to be conserved across taxa • Intron-exon genomic organization: The distinctive feature of eukaryotic genomes that sharply separates them from prokaryotic genomes is the presence of spliceosomal introns that interrupt protein-coding genes Small vs. Large Genomes 1. Compact, relatively small genomes of viruses, archaea, bacteria (typically, <10Mb), and many unicellular eukaryotes (typically, <20 Mb). In these genomes, protein-coding and RNA-coding sequences occupy most of the genomic sequence. 2. Expansive, large genomes of multicellular and some unicellular eukaryotes (typically, >100 Mb). In these genomes, the majority of the nucleotide sequence is non-coding. -

Transduction by Bacteriophage Ti * Henry Drexler
Proceedings of the National Academy of Sciences Vol. 66, No. 4, pp. 1083-1088, August 1970 Transduction by Bacteriophage Ti * Henry Drexler DEPARTMENT OF MICROBIOLOGY, BOWMAN GRAY SCHOOL OF MEDICINE, WAKE FOREST UNIVERSITY, WINSTON-SALEM, NORTH CAROLINA Communicated by Edward L. Tatum, May 25, 1970 Abstract. Amber mutants of the virulent coliphage T1 are able to transduce a wide variety of genetic characteristics from permissive to nonpermissive K strains of Escherichia coli. The virulent coliphage T1 is not known to be related to any temperate phage. Certain of the characteristics of T1 seem to be incompatible with its potential existence as a temperate phage; for example, the average latent period for T1 is only 13 min' and about 70% of its DNA is derived from the host.2 In order to demonstrate transduction by T1 it is necessray to provide condi- tions in which potential transductants are able to survive; this is accomplished by using amber mutants of T1 to transduce nonpermissive recipients. Experi- ments which show that T1 is a transducing phage are presented and have been designed chiefly to illustrate the following points: (1) Infection by T1 is able to cause heritable changes in recipients; (2) the genotype of the donor host is im- portant in determining what characteristics can be transferred to recipients; (3) the changes in the recipients are not caused by transformation; and (4) there is a similarity between the transducing activity and the plaque-forming ability of T1 with respect to serology, host range, and density. Materials and Methods. Bacterial strains: The abbreviations and symbols of Demerec et al.3 and Taylor and Trotter4 are used to describe all pertinent genotypes. -

Bacterial Genetics
BACTERIAL GENETICS Genetics is the study of genes including the structure of genetic materials, what information is stored in the genes, how the genes are expressed and how the genetic information is transferred. Genetics is also the study of heredity and variation. The arrangement of genes within organisms is its genotype and the physical characteristics an organism based on its genotype and the interaction with its environment, make up its phenotype. The order of DNA bases constitutes the bacterium's genotype. A particular organism may possess alternate forms of some genes. Such alternate forms of genes are referred to as alleles. The cell's genome is stored in chromosomes, which are chains of double stranded DNA. Genes are sequences of nucleotides within DNA that code for functional proteins. The genetic material of bacteria and plasmids is DNA. The two essential functions of genetic material are replication and expression. Structure of DNA The DNA molecule is composed of two chains of nucleotides wound around each other in the form of “double helix”. Double-stranded DNA is helical, and the two strands in the helix are antiparallel. The backbone of each strand comprises of repeating units of deoxyribose and phosphate residue. Attached to the deoxyribose is purine (AG) or pyrimidine (CT) base. Nucleic acids are large polymers consisting of repeating nucleotide units. Each nucleotide contains one phosphate group, one deoxyribose sugar, and one purine or pyrimidine base. In DNA the sugar is deoxyribose; in RNA the sugar is ribose. The double helix is stabilized by hydrogen bonds between purine and pyrimidine bases on the opposite strands. -

Evidence That Tolc Is Required for Functioning of the Mar/Acrab Efffux
JOURNAL OF BACTERIOLOGY, Oct. 1996, p. 5803–5805 Vol. 178, No. 19 0021-9193/96/$04.0010 Copyright q 1996, American Society for Microbiology Evidence that TolC Is Required for Functioning of the Mar/AcrAB Efflux Pump of Escherichia coli JOE A. FRALICK* Department of Microbiology and Immunology, Texas Tech University Health Sciences Center, Lubbock, Texas 79403 Received 10 June 1996/Accepted 16 July 1996 A study examining the influence of TolC on AcrA, AcrR, and MarR1 mutants indicates that functional TolC is required for the operation of the AcrAB efflux system and for the expression of the Mar phenotype. That the effect of TolC on the AcrAB pump is not regulatory in nature is shown by studies measuring the influence of a tolC::Tn10 insertion mutation on the expression of an acrA::lacZ reporter fusion. These results are compatible with the hypothesis that TolC is a component of the AcrAB efflux complex. Enteric bacteria have evolved to survive in an environment the heptose I phosphate of TolC LPS may be blocked by a rich with pernicious agents, such as bile salts, detergents, and phosphorylethanolamine group (32), it has been suggested that fatty acids. Protection against these inhibitors is due, in part, to the hypersusceptibility seen in TolC mutants is the result of the the selective permeability barrier of an outer membrane, which inability of TolC LPS to form a highly ordered LPS monolayer serves as the first line of defense against the rapid intrusion of (32). There are, however, significant differences between the lipophilic compounds (2, 11, 24, 25, 27). -
Recombination in Bacteria
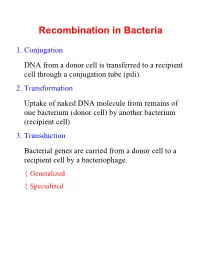
Recombination in Bacteria 1. Conjugation DNA from a donor cell is transferred to a recipient cell through a conjugation tube (pili). 2. Transformation Uptake of naked DNA molecule from remains of one bacterium (donor cell) by another bacterium (recipient cell). 3. Transduction Bacterial genes are carried from a donor cell to a recipient cell by a bacteriophage. { Generalized { Specialized Conjugation Ability to conjugate located on the F-plasmid F+ Cells act as donors F- Cells act as recipients F+/F- Conjugation: { F Factor “replicates off” a single strand of DNA. { New strand goes through pili to recipient cell. { New strand is made double stranded. { If entire F-plasmid crosses, then recipient cell becomes F+, otherwise nothing happens Conjugation with Hfr Hfr cell (High Frequency Recombination) cells have F-plasmid integrated into the Chromosome. Integration into the Chromosome is unique for each F-plasmid strain. When F-plasmid material is replicated and sent across pili, Chromosomal material is included. (Figure 6.10 in Klug & Cummings) When chromosomal material is in recipient cell, recombination can occur: { Recombination is double stranded. { Donor genes are recombined into the recipient cell. { Corresponding genes from recipient cell are recombined out of the chromosome and reabsorbed by the cell. Interrupted Mating Mapping 1. Allow conjugation to start Genes closest to the origin of replication site (in the direction of replication) are moved through the pili first. 2. After a set time, interrupt conjugation Only those genes closest to the origin of replication site will conjugate. The long the time, the more that is able to conjugate. 3. -

Transposon Insertion Mutagenesis in Mice for Modeling Human Cancers: Critical Insights Gained and New Opportunities
International Journal of Molecular Sciences Review Transposon Insertion Mutagenesis in Mice for Modeling Human Cancers: Critical Insights Gained and New Opportunities Pauline J. Beckmann 1 and David A. Largaespada 1,2,3,4,* 1 Department of Pediatrics, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] 2 Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA 3 Department of Genetics, Cell Biology and Development, University of Minnesota, Minneapolis, MN 55455, USA 4 Center for Genome Engineering, University of Minnesota, Minneapolis, MN 55455, USA * Correspondence: [email protected]; Tel.: +1-612-626-4979; Fax: +1-612-624-3869 Received: 3 January 2020; Accepted: 3 February 2020; Published: 10 February 2020 Abstract: Transposon mutagenesis has been used to model many types of human cancer in mice, leading to the discovery of novel cancer genes and insights into the mechanism of tumorigenesis. For this review, we identified over twenty types of human cancer that have been modeled in the mouse using Sleeping Beauty and piggyBac transposon insertion mutagenesis. We examine several specific biological insights that have been gained and describe opportunities for continued research. Specifically, we review studies with a focus on understanding metastasis, therapy resistance, and tumor cell of origin. Additionally, we propose further uses of transposon-based models to identify rarely mutated driver genes across many cancers, understand additional mechanisms of drug resistance and metastasis, and define personalized therapies for cancer patients with obesity as a comorbidity. Keywords: animal modeling; cancer; transposon screen 1. Transposon Basics Until the mid of 1900’s, DNA was widely considered to be a highly stable, orderly macromolecule neatly organized into chromosomes. -

The Acinetobacter Baumannii Mla System and Glycerophospholipid
RESEARCH ARTICLE The Acinetobacter baumannii Mla system and glycerophospholipid transport to the outer membrane Cassandra Kamischke1, Junping Fan1, Julien Bergeron2,3, Hemantha D Kulasekara1, Zachary D Dalebroux1, Anika Burrell2, Justin M Kollman2, Samuel I Miller1,4,5* 1Department of Microbiology, University of Washington, Seattle, United States; 2Department of Biochemistry, University of Washington, Seattle, United States; 3Department of Molecular Biology and Biotechnology, The University of Sheffield, Sheffield, United Kingdom; 4Department of Genome Sciences, University of Washington, Seattle, United States; 5Department of Medicine, University of Washington, Seattle, United States Abstract The outer membrane (OM) of Gram-negative bacteria serves as a selective permeability barrier that allows entry of essential nutrients while excluding toxic compounds, including antibiotics. The OM is asymmetric and contains an outer leaflet of lipopolysaccharides (LPS) or lipooligosaccharides (LOS) and an inner leaflet of glycerophospholipids (GPL). We screened Acinetobacter baumannii transposon mutants and identified a number of mutants with OM defects, including an ABC transporter system homologous to the Mla system in E. coli. We further show that this opportunistic, antibiotic-resistant pathogen uses this multicomponent protein complex and ATP hydrolysis at the inner membrane to promote GPL export to the OM. The broad conservation of the Mla system in Gram-negative bacteria suggests the system may play a conserved role in OM biogenesis. The importance of the Mla system to Acinetobacter baumannii OM integrity and antibiotic sensitivity suggests that its components may serve as new antimicrobial *For correspondence: therapeutic targets. [email protected] DOI: https://doi.org/10.7554/eLife.40171.001 Competing interests: The authors declare that no competing interests exist. -

Ribozyme-Mediated, Multiplex CRISPR Gene Editing and Crispri in Plasmodium Yoelii
bioRxiv preprint doi: https://doi.org/10.1101/481416; this version posted November 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Ribozyme-Mediated, Multiplex CRISPR Gene Editing and CRISPRi in Plasmodium yoelii Michael P. Walker1 and Scott E. Lindner1 * 1Department of Biochemistry and Molecular Biology, the Huck Center for Malaria Research, Pennsylvania State University, University Park, PA. *Correspondence: Scott E. Lindner, [email protected] Running Title: CRISPR-RGR in Plasmodium yoelii Keywords: Plasmodium, CRISPR, CRISPRi, Ribozyme, HDR, ALBA bioRxiv preprint doi: https://doi.org/10.1101/481416; this version posted November 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Abstract 2 Functional characterization of genes in Plasmodium parasites often relies on genetic 3 manipulations to disrupt or modify a gene-of-interest. However, these approaches are limited by 4 the time required to generate transgenic parasites for P. falciparum and the availability of a 5 single drug selectable marker for P. yoelii. In both cases, there remains a risk of disrupting native 6 gene regulatory elements with the introduction of exogenous sequences. To address these 7 limitations, we have developed CRISPR-RGR, a SpCas9-based gene editing system for 8 Plasmodium that utilizes a Ribozyme-Guide-Ribozyme (RGR) sgRNA expression strategy. 9 Using this system with P. yoelii, we demonstrate that both gene disruptions and coding sequence 10 insertions are efficiently generated, producing marker-free and scar-free parasites with homology 11 arms as short as 80-100bp. -

Double-Stranded RNA Killer Plasmid Replication in Saccharomyces Cerevisiae (Ski Mutants/Mak Mutants) Akio TOH-E* and REED B
Proc. Natl. Acad. Sci. USA Vol. 77, No. 1, pp. 527-530, January 1980 Genetics "Superkiller" mutations suppress chromosomal mutations affecting double-stranded RNA killer plasmid replication in Saccharomyces cerevisiae (ski mutants/mak mutants) AKIo TOH-E* AND REED B. WICKNERt Laboratory of Biochemical Pharmacology, National Institute of Arthritis, Metabolism, and Digestive Diseases, National Institutes of Health, Bethesda, Maryland 20205 Communicated by G. Gilbert Ashwell, October 17,1979 ABSTRACT Saccharomyces cerevisiae strains carrying a MATERIALS AND METHODS 1.5 X 106-dalton double-stranded RNA genome in virus-like particles (killer plasmid) secrete a protein toxin that kills strains Strains. Some of the strains of Saccharomyces cerevsiae used not carrying this plasmid. At least 28 chromosomal genes (mak in this study are listed in Table 1. Description of the phenotype genes) are required to maintain or replicate this plasmid. Re- and genotype of killer strains was presented previously (21). cessive mutations in any of four other chromosomal genes (ski Curing of the killer plasmid is done by growing killer strains for superkiller) result in enhanced toxin production. We report at an elevated temperature (37°C) (23). Mitochondrial DNA that many ski- mak- double mutants are able to maintain the killer plasmid, indicating that the SKIproducts have an effect was eliminated from strains by streaking to single colonies on on plasmid replication. The skil-) mutation suppresses (by- YPAD medium containing ethidium bromide at 30 ug/ml passes) all mak mutations tested except makl6-l. A variant killer (24). plasmid is described that confers the superkiller phenotype and, Media. YPAD, YPG, SD, presporulation medium, sporula- like chromosomal ski mutations, makes several mak genes tion medium, MB medium, and various omission media were dispensable for plasmid replication. -

Indirect Selection Against Antibiotic Resistance Via Specialized Plasmid-Dependent Bacteriophages
microorganisms Perspective Indirect Selection against Antibiotic Resistance via Specialized Plasmid-Dependent Bacteriophages Reetta Penttinen 1,2 , Cindy Given 1 and Matti Jalasvuori 1,* 1 Department of Biological and Environmental Science and Nanoscience Center, University of Jyväskylä, Survontie 9C, P.O.Box 35, FI-40014 Jyväskylä, Finland; reetta.k.penttinen@jyu.fi (R.P.); cindy.j.given@jyu.fi (C.G.) 2 Department of Biology, University of Turku, FI-20014 Turku, Finland * Correspondence: matti.jalasvuori@jyu.fi; Tel.: +358-504135092 Abstract: Antibiotic resistance genes of important Gram-negative bacterial pathogens are residing in mobile genetic elements such as conjugative plasmids. These elements rapidly disperse between cells when antibiotics are present and hence our continuous use of antimicrobials selects for elements that often harbor multiple resistance genes. Plasmid-dependent (or male-specific or, in some cases, pilus-dependent) bacteriophages are bacterial viruses that infect specifically bacteria that carry certain plasmids. The introduction of these specialized phages into a plasmid-abundant bacterial community has many beneficial effects from an anthropocentric viewpoint: the majority of the plasmids are lost while the remaining plasmids acquire mutations that make them untransferable between pathogens. Recently, bacteriophage-based therapies have become a more acceptable choice to treat multi-resistant bacterial infections. Accordingly, there is a possibility to utilize these specialized phages, which are not dependent on any particular pathogenic species or strain but rather on the resistance-providing elements, in order to improve or enlengthen the lifespan of conventional antibiotic approaches. Here, Citation: Penttinen, R.; Given, C.; we take a snapshot of the current knowledge of plasmid-dependent bacteriophages.