Report on Huntsman's Cast Steel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MS452 Title: Archives of Cultural Tradition Miscellaneous
University of Sheffield Library. Special Collections and Archives Ref: MS452 Title: Archives of Cultural Tradition Miscellaneous Manuscripts Scope: An extremely wide and varied collection of material relating to mainly British cultural tradition. The collection is loosely focused on folklore, dialect and domesticity. It is made up of printed ephemera, hand written accounts and reproduced and original documents across a wide time frame. Dates: 1771-1999 Level: Collection Extent: 64 boxes Name of creator: Archives of Cultural Tradition Administrative / biographical history: This collection is made up of individual donations to the Archives of Cultural Tradition. Items mainly relate to British cultural tradition, although other countries are present. Folklore, dialect and domesticity are represented through printed ephemera, hand-written accounts and published documents. Much of the material covers local history and folk-traditions with newspaper cuttings and relevant articles as well as survey studies collected by the Archives of Cultural Tradition. Source: Donated between 1963 and 1999; transferred to University of Sheffield Library July 2008 System of arrangement: As received Subjects: Folklore, Cultural traditions Conditions of access: Available to all researchers, by appointment Restrictions: None Copyright: According to document Finding aids: Listed MS452 Archives of Cultural Traditions Miscellaneous Manuscripts 1.1. Sykes and Barron Ballad Roll, photocopy. Unknown donor, unknown date 1.2. Student Selected Study, Ian D Hunter, post graduate, ”The Centre for English Cultural Tradition and Language” Photocopy, 1986. Unknown donor, unknown date 1.3. Jean Massey collection - articles, photographs and books. Jean Massey donor A. Article re Marjory Fraser, “Songs of the Hebrides” From Scottish Field, November 1957. B. -

A Sheffield Hallam University Thesis
Business, training and education: Sheffield circa 1880-1940 EASON, Mark Available from the Sheffield Hallam University Research Archive (SHURA) at: http://shura.shu.ac.uk/3097/ A Sheffield Hallam University thesis This thesis is protected by copyright which belongs to the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Please visit http://shura.shu.ac.uk/3097/ and http://shura.shu.ac.uk/information.html for further details about copyright and re-use permissions. 100369555 8 Sheffield Hallam University REFERENCE ONLY SHEFFIELD HALLAM » s r r y i £ A R N W 0 CENTO COLLEGIATE CRESCENT SHEFFIELD SIO 2BP ProQuest Number: 10700839 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10700839 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 Business, Training and Education: -

There's Metal in Those Hills Sheffield Was Once the Iron, Steel and Cutlery
There’s Metal in Those Hills Sheffield was once the iron, steel and cutlery capital of the world. The hills around Sheffield and Rotherham were full of raw materials like coal and iron ore that could be used in cutlery and blade production. Before we were Famous Sheffield and Rotherham became famous for manufacturing and production for four main reasons: crucible steel, stainless steel, electroplating and cutlery. Crucible Steel Benjamin Huntsman invented the crucible steel process. Before this process was invented, the quality of the steel was unreliable and slow to produce. Benjamin Huntsman’s invention allowed people to produce tougher, high-quality steel in larger quantities. Stainless Steel Harry Brearley began testing why rifles rusted and exploring ways to stop steel from rusting. He developed Stainless Steel which was much more rust resistant than the steel that had been used previously. Cutlery Sheffield and Rotherham have been connected to the production of cutlery since the 1600s. Sheffield was the main center of cutlery production in England outside of London. Sheffield became world famous for its production of high-quality cutlery. The moving story of Sheffield’s remarkable Women of Steel Yorkshire Post article 13th June 2020 The story of Kathleen, one of the last surviving Sheffield steelworkers from the war. When war broke out, the lives of the young women of Sheffield were turned upside down. With the men sent away to fight they had no choice but to step into their shoes and became the backbone of the city’s steel industry. Through hard graft and companionship in the gruelling, and often dangerous world of factory work, they vowed to keep the foundry fires burning. -

The Economic Development of Sheffield and the Growth of the Town Cl740-Cl820
The Economic Development of Sheffield and the Growth of the Town cl740-cl820 Neville Flavell PhD The Division of Adult Continuing Education University of Sheffield February 1996 Volume One THE ECONOMIC DEVELOPMENT OF SHEFFIELD AND THE GROWTH OF THE TOWN cl740-c 1820 Neville Flavell February 1996 SUMMARY In the early eighteenth century Sheffield was a modest industrial town with an established reputation for cutlery and hardware. It was, however, far inland, off the main highway network and twenty miles from the nearest navigation. One might say that with those disadvantages its future looked distinctly unpromising. A century later, Sheffield was a maker of plated goods and silverware of international repute, was en route to world supremacy in steel, and had already become the world's greatest producer of cutlery and edge tools. How did it happen? Internal economies of scale vastly outweighed deficiencies. Skills, innovations and discoveries, entrepreneurs, investment, key local resources (water power, coal, wood and iron), and a rapidly growing labour force swelled largely by immigrants from the region were paramount. Each of these, together with external credit, improved transport and ever-widening markets, played a significant part in the town's metamorphosis. Economic and population growth were accompanied by a series of urban developments which first pushed outward the existing boundaries. Considerable infill of gardens and orchards followed, with further peripheral expansion overspilling into adjacent townships. New industrial, commercial and civic building, most of it within the central area, reinforced this second phase. A period of retrenchment coincided with the French and Napoleonic wars, before a renewed surge of construction restored the impetus. -
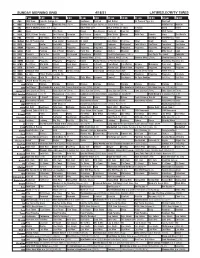
Sunday Morning Grid 4/18/21 Latimes.Com/Tv Times
SUNDAY MORNING GRID 4/18/21 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Face the Nation (N) News SunPower Dest LA Bull Riding 25 Years of Tiger (N) PGA Golf 4 NBC Today in LA Weekend Meet the Press (N) Å Hockey Washington Capitals at Boston Bruins. (N) IndyCar Pre IndyCar 5 CW KTLA 5 Morning News at 7 (N) Å KTLA News at 9 KTLA 5 News at 10am In Touch David Relief 7 ABC News This Week Ocean Sea Rescue Hearts of Free Ent. QB21 MLS Soccer 9 KCAL KCAL 9 News Sunday Joel Osteen Jeremiah Joel Osteen Jentzen Mike Webb Harvest Gold Coin Danette Icons The World’s 1 1 FOX PROTECT Jack Hibbs Fox News Sunday The Issue PBA Bowling Super Slam. (N) RaceDay NASCAR Cup Series 1 3 MyNet Bel Air Presbyterian Fred Jordan Freethought In Touch Jack Hibbs Paid Prog. Silver Shark News The Issue 1 8 KSCI Relief Dental SmileMO AAA Relief PROTECT Kenmore Bathroom? Paint Like A Can’tHear Transform Sex Abuse 2 2 KWHY Programa Programa Revitaliza Programa Programa Programa Programa Programa Programa Programa Programa Programa 2 4 KVCR Paint Painting Joy of Paint Wyland’s Paint This Painting Kitchen Mexican Nick Lidia Milk Street Cook 2 8 KCET Darwin’s Cat in the SciGirls Odd Squad Cyberchase Biz Kid$ Great Performances (TVG) Å Easy Yoga: The Secret Colorado 3 0 ION Law & Order (TV14) Law & Order (TV14) Criminal Minds (TV14) Criminal Minds (TV14) Criminal Minds (TV14) Criminal Minds (TV14) 3 4 KMEX Conexión Programa Programa Programa Fútbol Fútbol Mexicano Primera División (N) República Deportiva (N) 4 0 KTBN R. -

South Yorkshire
INDUSTRIAL HISTORY of SOUTH RKSHI E Association for Industrial Archaeology CONTENTS 1 INTRODUCTION 6 STEEL 26 10 TEXTILE 2 FARMING, FOOD AND The cementation process 26 Wool 53 DRINK, WOODLANDS Crucible steel 27 Cotton 54 Land drainage 4 Wire 29 Linen weaving 54 Farm Engine houses 4 The 19thC steel revolution 31 Artificial fibres 55 Corn milling 5 Alloy steels 32 Clothing 55 Water Corn Mills 5 Forging and rolling 33 11 OTHER MANUFACTUR- Windmills 6 Magnets 34 ING INDUSTRIES Steam corn mills 6 Don Valley & Sheffield maps 35 Chemicals 56 Other foods 6 South Yorkshire map 36-7 Upholstery 57 Maltings 7 7 ENGINEERING AND Tanning 57 Breweries 7 VEHICLES 38 Paper 57 Snuff 8 Engineering 38 Printing 58 Woodlands and timber 8 Ships and boats 40 12 GAS, ELECTRICITY, 3 COAL 9 Railway vehicles 40 SEWERAGE Coal settlements 14 Road vehicles 41 Gas 59 4 OTHER MINERALS AND 8 CUTLERY AND Electricity 59 MINERAL PRODUCTS 15 SILVERWARE 42 Water 60 Lime 15 Cutlery 42 Sewerage 61 Ruddle 16 Hand forges 42 13 TRANSPORT Bricks 16 Water power 43 Roads 62 Fireclay 16 Workshops 44 Canals 64 Pottery 17 Silverware 45 Tramroads 65 Glass 17 Other products 48 Railways 66 5 IRON 19 Handles and scales 48 Town Trams 68 Iron mining 19 9 EDGE TOOLS Other road transport 68 Foundries 22 Agricultural tools 49 14 MUSEUMS 69 Wrought iron and water power 23 Other Edge Tools and Files 50 Index 70 Further reading 71 USING THIS BOOK South Yorkshire has a long history of industry including water power, iron, steel, engineering, coal, textiles, and glass. -

ARCHAEOLOGY DATASHEET 302 Steelmaking
ARCHAEOLOGY DATASHEET 302 Steelmaking Introduction A single forging produced ‘shear steel’, a second operation Although an important aspect of medieval and earlier resulted in ‘double shear steel’ and so-on. societies, the manufacture of steel was industrialised during Furnace design changed over time, and also appears to the post-medieval period. Many complementary techniques have shown some regional variation. The first English steel were developed which often operated at the same time on furnaces were built at Coalbrookdale (Shropshire) by Sir the same site; there were also close links with other Basil Brooke in c1615 and c1630. These were circular in ironworking processes. This datasheet describes pre-20th plan with a central flue. They probably contained a single century steelmaking processes in the UK, their material chest and would have had a conical chimney. Other 17th- remains and metallurgical potential. century cementation furnaces were located in and around Birmingham, Wolverhampton, Stourbridge and Bristol, but Carbon steel and other alloys none of these have been excavated. The north-east became Until the late 19th century, steel was, like other types of the main area of cementation steelmaking in the late 17th iron, simply an alloy of iron and carbon (HMS datasheet and early 18th centuries. Ambrose Crowley established 201). There was considerable variation in the nature of several cementation steelworks, and others followed. Of ‘steel’ and in the properties of individual artefacts. Cast these, the oldest standing structure is at Derwentcote iron, smelted in the blast furnace usually had a carbon (County Durham), built in 1734. Unlike the West Midlands content of 5-8%, making it tough but brittle. -

HMSNEWS Gill Juleff and Matthew Baker
Instrumenting an experimental Sri Lankan wind-driven furnace HMSNEWS Gill Juleff and Matthew Baker Historical Metallurgy Society In 2007 the Society’s Coghlan Bequest generously supported Matthew Baker, an Engineering student from 68 Spring 2008 Exeter University, who joined a team from the Archaeology Department at Exeter University, under the direction of Gill Juleff, travelling to Sri Lanka to Annual General Meeting carry out a second series of iron smelting experiments. Cambridge, June 2008 The funds awarded to Matthew were used to purchase thermocouples and scientific equipment to help record This year our AGM will be held in Cambridge on conditions within these highly efficient and unusual Saturday 14th June at the Scott Polar Research Institute. furnaces. The following report summarises the As usual the AGM will be held first to which all Monsoon Steel project and Matthew’s role in it, and members of the Society are welcome to attend. presents some preliminary results. A more Following the AGM we will have our usual Spring comprehensive account of the new experiments will be meeting of presentations and visits. published on completion of the full analysis of the data. Presentations will include: • Steve Walton, Penn State University, on Scientific In the early 1990’s research carried out in Sri Lanka Instruments uncovered evidence of a large-scale iron producing industry of the first millennium AD at Samanalawewa, • Robert Smith on Frobisher’s Gold. in the southern foothills of the Central Highlands. Extensive survey and excavations revealed that iron was We will also have the opportunity to see some of the smelted in shallow, elongated furnaces which were wonderful museums in Cambridge – The Scott Polar located at the tops of steep hillsides and were driven by Museum, The Fitzwilliam, the Sedgewick and the powerful dry monsoonal winds – a furnace design and Archaeological Museum. -

Untitled Approximate Original Scheduled (Eight Pages) On-Sale Date: July 11, 1978
TABLE OF CONTENTS Introduction and Acknowledgements ....................................................... 5 Prologue. 7 DC Comics’ Lineup of Titles: Early 1976 ................................................ 10 Part 1: Pre-Explosion (1976-1978) ........................................................ 11 Interlude: Ring Out the Old, Ring In the New ............................................ 23 DC Comics’ Lineup of Titles: Early 1977 ................................................ 31 DC Comics’ Lineup of Titles: Early 1978 (Pre-DC Explosion) .............................. 52 Part 2: Explosion (1978) ................................................................. 53 DC Comics’ Lineup of Titles: June, July and August 1978 (The DC Explosion) ............... 66 DC Comics’ Lineup of Titles: June, July and August 1978 (Unpublished) .................... 66 Part 3: Implosion (1978-1980) ............................................................ 67 DC Comics’ Lineup of Titles: Early 1979 (Post-DC Implosion) ............................. 76 Bonus Gallery ....................................................................... 79 Interlude: Cancelled Comic Cavalcade: The Index ........................................ 90 Interlude: Whatever Happened to –? ................................................... 98 DC Comics’ Lineup of Titles: June, July and August 1980 ................................ 117 Cancellations by Month of Publication ................................................... 127 Afterword ........................................................................... -

Sheffield City Council
SHEFFIELD CITY COUNCIL - BUILDINGS AT RISK BY CATEGORY No Name G Last/Current Notes Use RELIGIOUS BUILDINGS/CEMETERY STRUCTURES 1 Roman Catholic mortuary chapel, City Road cemetery II Chapel 2 Blitz grave, City Road cemetery II Monument 3 Tomb of Benjamin Huntsman, Attercliffe Chapel, Attercliffe Common II Monument 4 Salvation Army Citadel, Cross Burgess Street II Religious Part of the New Retail Vacant Quarter. Appeal lodged against refusal of lbc for conversion. 5 St Silas Church II Religious LBC granted for conversion Vacant to offices and health centre 2005 6 Anglican Chapel, SGC II Religious LBC refused 2007 – lack of Vacant information 7 Non Conformist Chapel, SGC II Religious Leased to FOSGC Vacant 8 Catacombs at SGC II* Monument Leased to FOSGC 9 Loxley Chapel II* Religious Pre-app meeting held. Vacant Application expected 09 10 Crookes Valley Methodist Church II Religious LBC application received Vacant Dec 2007. To be monitored. 11 James Nicholson memorial, SGC II Monument 12 Former Middlewood Hospital Church II Religious To be monitored Vacant Needs new use. 13 Walsh Monument, Rivelin Cemetery II Monument METAL TRADES BUILDINGS 14 286 Coleridge Road, Crucible steel melting shop II Workshop 15 East range, Cornish Place Works, Cornish Street II Workshop Awaiting repairs by owner. Pre-app meetings ongoing. 16 Darnell Works south workshop II* Workshop Darnell Works – south east workshop II* Darnell Works - weighbridge II Darnell Works - offices II 17 Grinding hull, forge, assembly shop. 120a Broomspring Lane II Workshop LBC granted -

Comics' Bronze Age and Beyond!
COMICS’ BRONZE AGE AND BEYOND! A pril 201 5 No.79 $ 8 . 9 5 Captain Atom and the Ghost TM & © DC Comics. All Rights Reserved. 0 3 1 82658 27762 8 FROM CHARLTON TO DC: ACTION HEROES IN THE BRONZE AGE Blue Beetle • Captain Atom • Peacemaker • Thunderbolt • Blockbuster Weekly • Dave Gibbons Watchmen interview with Bates • Broderick • Collins • Cullins • Kupperberg • Wein & more Volume 1, Number 79 April 2015 Celebrating the Best Comics of the '70s, '80s, Comics’ Bronze Age and Beyond! '90s, and Beyond! EDITOR-IN-CHIEF Michael Eury PUBLISHER John Morrow DESIGNER Rich Fowlks COVER ARTIST Allen Milgrom COVER COLORIST Glenn Whitmore COVER DESIGNER Michael Kronenberg PROOFREADER Rob Smentek SPECIAL THANKS Michael Ambrose James Kingman Cary Bates Ted Kord Bill Black Paul Kupperberg Pat Broderick Kevin Maguire Michael Browning Robert Menzies Mike Collins Allen Milgrom Comic Book Resources Elizabeth Millsted Corpus ChrisTi College Ian Millsted BACK SEAT DRIVER: The Action Heroes: They’re Not Half-Bad . .2 Cambridge Dennis O’Neil A quick history of Charlton Comics and its hodgepodge-heroes Paris Cullins Bob Rozakis Andrew Czekalski Tod Smith FLASHBACK: Inaction Heroes . .12 DC Comics Anthony Snyder Charlton Spotlight editor Michael Ambrose examines the Action Heroes’ pre–DC purgatory Daniel DeAngelo Andrew Standish Michael Dunne Roy Thomas GREATEST STORIES NEVER TOLD: Blockbuster Weekly . .18 Scott Dutton Len Wein Bob Greenberger goes behind the scenes of DC’s aborted Action Heroes revival Excelsior Comics Greg Weisman T. C. Ford John Wells FLASHBACK: Re-Meet the Beetle . .23 Dave Gibbons Michael Zeno The Blue Beetle is rebooted at DC Comics Grand Comics Database Dedicated to the FLASHBACK: Quantum Entanglements: Captain Atom’s Rebirth in the 1980s . -
Gold Check Gold Check
PAID Stk# 7758 Stk# 5853 per mo.* ECRWSS Eagle River PRSRT STD PRSRT U.S. Postage Permit No. 13 POSTAL PATRON POSTAL per mo.* Only 27,000 miles 2010 Toyota Tacoma SR5 Tacoma 423 $ 421 $ Outdoorsman Quad 2012 Dodge Ram 1500 per mo.* Stk# 9476 Saturday, Saturday, Rav4 4WD per mo.* (715) 479-4421 March 14, 2015 14, March 2010 Toyota 292 $ Stk# 7692 AND THE THREE LAKES NEWS 2008 Dodge Plus tax, title, license and dealer doc fee. $2,000 down or trade-in approved credit, 3.9% for 60 months *To approved credit, 3.9% for 48 months **To See Parsons for complete details. ***Limitations apply. 245 $ Grand Caravan SXT per mo.* Check Check G6 GT Only 23,000 miles 2009 Pontiac Stk# 0259 218 $ Stk# 3438 per mo.* A SPECIAL SECTION OF THE VILAS COUNTY NEWS-REVIEW THE VILAS COUNTY SECTION OF SPECIAL A 2010 Chevy —CERTIFIED— —CERTIFIED— Malibu 2LT V6 Malibu 2LT 212 Stk# 6529 $ per mo.** Gold Gold 2006 Mercury Milan Premier 134 $ 5353 Hwy. 70 West 5353 Hwy. (800) 341-4421 Stk# 7663 Eagle River, WI 54521 Eagle River, per mo.* Impala LT per mo.* Every Certified Program Vehicle must pass a rigorous quality assurance inspection Every Certified Program Vehicle 2011 Chevy 2011 including coverage in the event you are locked out of your vehicle, have a flat tire, need jump start 195 $ 2011 GMC 2011 Sierra Ext Cab 12-month, 12,000-mile Limited Warranty, Towing Coverage, Trip Interruption Coverage, Rental Car Coverage Trip Coverage, Towing 12-month, 12,000-mile Limited Warranty, 322 of Eagle River $ Advantages of a Parsons Eagle River Gold Check Certified Preowned Vehicle:*** Stk# 8439 Stk# 3047 NORTH WOODS NORTH THE PAUL BUNYAN OF NORTH WOODS ADVERTISING WOODS OF NORTH BUNYAN THE PAUL per mo.* “Striving to best do for what’s you!” per mo.** Cruze 2011 Chevy 2011 or run out of gas 2006 Ford Mustang V6 184 •Assistance Roadside Plan • Higher Standards Vehicle - • - Limited Warranty $ 180 Parsons Parsons Stk# 6875 $ © Eagle River These vehicles and more are… Publications, Inc.