Supporting Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 231 _____________________________ _____________________________ Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21 BY JOHAN BYLUND ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2000 Dissertation for the Degree of Doctor of Philosophy (Faculty of Pharmacy) in Pharmaceutical Pharmacology presented at Uppsala University in 2000 ABSTRACT Bylund, J. 2000. Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid: Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from Faculty of Pharmacy 231 50 pp. Uppsala. ISBN 91-554-4784-8. Cytochrome P450 (P450 or CYP) is an enzyme system involved in the oxygenation of a wide range of endogenous compounds as well as foreign chemicals and drugs. This thesis describes investigations of P450-catalyzed oxygenation of prostaglandins, linoleic and arachidonic acids. The formation of bisallylic hydroxy metabolites of linoleic and arachidonic acids was studied with human recombinant P450s and with human liver microsomes. Several P450 enzymes catalyzed the formation of bisallylic hydroxy metabolites. Inhibition studies and stereochemical analysis of metabolites suggest that the enzyme CYP1A2 may contribute to the biosynthesis of bisallylic hydroxy fatty acid metabolites in adult human liver microsomes. 19R-Hydroxy-PGE and 20-hydroxy-PGE are major components of human and ovine semen, respectively. They are formed in the seminal vesicles, but the mechanism of their biosynthesis is unknown. Reverse transcription-polymerase chain reaction using degenerate primers for mammalian CYP4 family genes, revealed expression of two novel P450 genes in human and ovine seminal vesicles. -

Synonymous Single Nucleotide Polymorphisms in Human Cytochrome
DMD Fast Forward. Published on February 9, 2009 as doi:10.1124/dmd.108.026047 DMD #26047 TITLE PAGE: A BIOINFORMATICS APPROACH FOR THE PHENOTYPE PREDICTION OF NON- SYNONYMOUS SINGLE NUCLEOTIDE POLYMORPHISMS IN HUMAN CYTOCHROME P450S LIN-LIN WANG, YONG LI, SHU-FENG ZHOU Department of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing 100191, P. R. China (LL Wang & Y Li) Discipline of Chinese Medicine, School of Health Sciences, RMIT University, Bundoora, Victoria 3083, Australia (LL Wang & SF Zhou). 1 Copyright 2009 by the American Society for Pharmacology and Experimental Therapeutics. DMD #26047 RUNNING TITLE PAGE: a) Running title: Prediction of phenotype of human CYPs. b) Author for correspondence: A/Prof. Shu-Feng Zhou, MD, PhD Discipline of Chinese Medicine, School of Health Sciences, RMIT University, WHO Collaborating Center for Traditional Medicine, Bundoora, Victoria 3083, Australia. Tel: + 61 3 9925 7794; fax: +61 3 9925 7178. Email: [email protected] c) Number of text pages: 21 Number of tables: 10 Number of figures: 2 Number of references: 40 Number of words in Abstract: 249 Number of words in Introduction: 749 Number of words in Discussion: 1459 d) Non-standard abbreviations: CYP, cytochrome P450; nsSNP, non-synonymous single nucleotide polymorphism. 2 DMD #26047 ABSTRACT Non-synonymous single nucleotide polymorphisms (nsSNPs) in coding regions that can lead to amino acid changes may cause alteration of protein function and account for susceptivity to disease. Identification of deleterious nsSNPs from tolerant nsSNPs is important for characterizing the genetic basis of human disease, assessing individual susceptibility to disease, understanding the pathogenesis of disease, identifying molecular targets for drug treatment and conducting individualized pharmacotherapy. -

Essential Role of the Cytochrome P450 CYP4F22 in the Production of Acylceramide, the Key Lipid for Skin Permeability Barrier Formation
Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation Yusuke Ohnoa, Shota Nakamichia, Aya Ohkunia, Nozomi Kamiyamaa, Ayano Naoeb, Hisashi Tsujimurab, Urara Yokoseb, Kazumitsu Sugiurac, Junko Ishikawab, Masashi Akiyamac, and Akio Kiharaa,1 aLaboratory of Biochemistry, Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo 060-0812, Japan; bKao Corporation, Haga-gun, Tochigi 321-3497, Japan; and cDepartment of Dermatology, Nagoya University Graduate School of Medicine, Showa-ku, Nagoya 466-8550, Japan Edited by David W. Russell, University of Texas Southwestern Medical Center, Dallas, TX, and approved May 21, 2015 (received for review February 19, 2015) A skin permeability barrier is essential for terrestrial animals, and its lamellae and/or to stabilize the multiple lipid layers. Linoleic impairment causes several cutaneous disorders such as ichthyosis and acid is one of the essential FAs, and its deficiency causes ich- atopic dermatitis. Although acylceramide is an important lipid for the thyosis symptoms resulting from a failure to form normal acyl- skin permeability barrier, details of its production have yet to be de- ceramide (8). Ichthyosis is a cutaneous disorder accompanied termined, leaving the molecular mechanism of skin permeability bar- by dry, thickened, and scaly skin; it is caused by a barrier ab- rier formation unclear. Here we identified the cytochrome P450 gene normality. In patients who have atopic dermatitis, both total CYP4F22 (cytochrome P450, family 4, subfamily F, polypeptide 22) as ceramide levels and the chain length of ceramides are de- the long-sought fatty acid ω-hydroxylase gene required for acylcer- creased, and ceramide composition is altered also (9–11). -

Effect of Genetic Variability in the CYP4F2, CYP4F11 and CYP4F12 Genes on Liver Mrna Levels and Warfarin Response
Effect of genetic variability in the CYP4F2, CYP4F11 and CYP4F12 genes on liver mRNA levels and warfarin response J. Eunice Zhang1, Kathrin Klein2, Andrea L. Jorgensen3, Ben Francis3, Ana Alfirevic1, Stephane Bourgeois4, Panagiotis Deloukas4,5,6, Ulrich M. Zanger2 and Munir Pirmohamed1* 1Wolfson Centre for Personalized Medicine, Department of Molecular & Clinical Pharmacology, The University of Liverpool, UK 2Dr Margarete Fischer-Bosch Institute of Clinical Pharmacology, Stuttgart, and University of Tuebingen, Tuebingen, Germany. 3Department of Biostatistics, The University of Liverpool, UK 4William Harvey Research Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, UK 5Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK. 6Princess Al-Jawhara Al-Brahim Centre of Excellence in Research of Hereditary Disorders, King Abdulaziz University, Jeddah, Saudi Arabia. Correspondence: Professor Sir Munir Pirmohamed Wolfson Centre for Personalised Medicine Department of Molecular & Clinical Pharmacology Block A: Waterhouse Buildings The University of Liverpool 1-5 Brownlow Street L69 3GL, UK [email protected] Running Title: Genotype-phenotype assessment of CYP4F12-CYP4F2-CYP4F11 region Keywords: warfarin, pharmacogenetics, mRNA expression, CYP4F2, CYP4F11, CYP4F12 1 Abstract Genetic polymorphisms in the gene encoding cytochrome P450 (CYP) 4F2, a vitamin K oxidase, affect stable warfarin dose requirements and time to therapeutic INR. CYP4F2 is part of the CYP4F gene cluster, which is highly polymorphic and exhibits a high degree of linkage disequilibrium, making it difficult to define causal variants. Our objective was to examine the effect of genetic variability in the CYP4F gene cluster on expression of the individual CYP4F genes and warfarin response. mRNA levels of the CYP4F gene cluster were quantified in human liver samples (n=149) obtained from a well characterized liver bank and fine mapping of the CYP4F gene cluster encompassing CYP4F2, CYP4F11 and CYP4F12 was performed. -
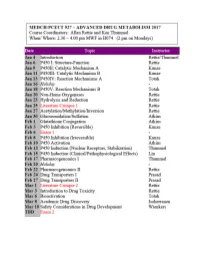
CYTOCHROME P450: Structure-Function
MEDCH 527 AER Jan. 4-6, 2017 CYTOCHROME P450: Structure-Function 1. General P450 Characteristics anD Taxonomy 2. Human P450s – Substrate anD Inhibitor Selectivities 3. Structure-Function Aspects of LiganD BinDing, P450 ReDuction anD Oxygen Activation References P450 Homepage -http:// http://drnelson.uthsc.edu/CytochromeP450.html Nelson DR et al., Pharmacogenetics. 1996 6(1):1-42: Nelson DR et al., Pharmacogenetics. 2004 14(1):1-18. Testa, B. The Biochemistry of Drug Metabolism: A 6 Part Series in Chem. BioDivers. (2006-2008). Sligar, SG. Glimpsing the critical intermediate in cytochrome P450 oxidations. Science. 2010 Nov 12;330(6006):924-5. Johnson EF, et al. Correlating structure and function of drug metabolizing enzymes:Progress and ongoing challenges. Drug Metab. Dispos. 42:9-22 (2014). Zientek MA and Youdim K, Reaction Phenotyping:Advances in experimental strategies used to characterize the contribution of drug metabolizing enzymes. Drug Metab. Dispos. 43:163-181 (2015). Almazroo et al., Drug Metabolism in the Liver. Clin. Liver Dis. 21:1-20 (2017). Absorption, Distribution, Metabolism and Excretion (ADME) of Orally Administered Drugs To reach their sites of action in the body, orally administered drugs must be absorbed from the small intestine, survive first pass metabolism - typically in the liver - before eventually being excreted, usually as drug metabolites in the bile and kidney. Therefore, clinically useful drugs must be able to cross an array of cell membranes, which are composed of a lipid bilayer. Drugs must exhibit an adequate degree of lipophilicity (logP of ~2-4) in order to able to dissolve into this lipoidal environment. Many drug metabolism processes render lipophilic drugs more water-soluble so as to facilitate excretion via the kidneys and bile. -

Urinary Sediment Transcriptomic and Longitudinal Data to Investigate Renal Function Decline in Type 1 Diabetes
ORIGINAL RESEARCH published: 30 April 2020 doi: 10.3389/fendo.2020.00238 Urinary Sediment Transcriptomic and Longitudinal Data to Investigate Renal Function Decline in Type 1 Diabetes Maria Beatriz Monteiro 1, Tatiana S. Pelaes 1, Daniele P. Santos-Bezerra 1, Karina Thieme 2, Antonio M. Lerario 3, Sueli M. Oba-Shinjo 4, Ubiratan F. Machado 2, Marisa Passarelli 5,6, Suely K. N. Marie 4 and Maria Lúcia Corrêa-Giannella 1,6* 1 Laboratório de Carboidratos e Radioimunoensaio (LIM-18), Faculdade de Medicina, Hospital das Clinicas HCFMUSP, Universidade de São Paulo, São Paulo, Brazil, 2 Department of Physiology and Biophysics, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil, 3 Division of Metabolism, Endocrinology, and Diabetes, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States, 4 Laboratory of Molecular and Cellular Biology (LIM-15), Faculdade de Medicina, Hospital das Clinicas HCFMUSP, Universidade de São Paulo, São Paulo, Brazil, 5 Laboratório de Lípides (LIM-10), Faculdade de Medicina, Hospital das Clinicas HCFMUSP, Universidade de São Paulo, São Paulo, Brazil, 6 Programa de Pós-graduação em Medicina, Universidade Nove de Julho (UNINOVE), São Paulo, Brazil Edited by: Ondrejˇ Šeda, Introduction: Using a discovery/validation approach we investigated associations Charles University, Czechia between a panel of genes selected from a transcriptomic study and the estimated Reviewed by: glomerular filtration rate (eGFR) decline across time in a cohort of type 1 diabetes Ilse Sofia Daehn, Icahn School of Medicine at Mount (T1D) patients. Sinai, United States Tomislav Bulum, Experimental: Urinary sediment transcriptomic was performed to select highly University of Zagreb, Croatia modulated genes in T1D patients with rapid eGFR decline (decliners) vs. -

Biodiversity of P-450 Monooxygenase: Cross-Talk
Cytochrome P450: Oxygen activation and biodiversty 1 Biodiversity of P-450 monooxygenase: Cross-talk between chemistry and biology Heme Fe(II)-CO complex 450 nm, different from those of hemoglobin and other heme proteins 410-420 nm. Cytochrome Pigment of 450 nm Cytochrome P450 CYP3A4…. 2 High Energy: Ultraviolet (UV) Low Energy: Infrared (IR) Soret band 420 nm or g-band Mb Fe(II) ---------- Mb Fe(II) + CO - - - - - - - Visible region Visible bands Q bands a-band, b-band b a 3 H2O/OH- O2 CO Fe(III) Fe(II) Fe(II) Fe(II) Soret band at 420 nm His His His His metHb deoxy Hb Oxy Hb Carbon monoxy Hb metMb deoxy Mb Oxy Mb Carbon monoxy Mb H2O/Substrate O2-Substrate CO Substrate Soret band at 450 nm Fe(III) Fe(II) Fe(II) Fe(II) Cytochrome P450 Cys Cys Cys Cys Active form 4 Monooxygenase Reactions by Cytochromes P450 (CYP) + + RH + O2 + NADPH + H → ROH + H2O + NADP RH: Hydrophobic (lipophilic) compounds, organic compounds, insoluble in water ROH: Less hydrophobic and slightly soluble in water. Drug metabolism in liver ROH + GST → R-GS GST: glutathione S-transferase ROH + UGT → R-UG UGT: glucuronosyltransferaseGlucuronic acid Insoluble compounds are converted into highly hydrophilic (water soluble) compounds. 5 Drug metabolism at liver: Sleeping pill, pain killer (Narcotic), carcinogen etc. Synthesis of steroid hormones (steroidgenesis) at adrenal cortex, brain, kidney, intestine, lung, Animal (Mammalian, Fish, Bird, Insect), Plants, Fungi, Bacteria 6 NSAID: non-steroid anti-inflammatory drug 7 8 9 10 11 Cytochrome P450: Cysteine-S binding to Fe(II) heme is important for activation of O2. -

Characterisation of Equine Cytochrome P450s Catherine Orr
Characterisation of Equine Cytochrome P450s Catherine Orr, BSc, MRes Thesis submitted to the University of Nottingham for the degree of Doctor of Philosophy October 2015 Abstract Cytochrome P450s (CYPs) are a superfamily of enzymes involved in the phase I metabolism of endogenous and exogenous substances. They are present in almost all forms of life and have been studied extensively, particularly in relation to human medicine, where knowledge of their activities is essential for predicting drug-drug interactions. In the horse, little is currently known about CYP-specific drug metabolism, which holds importance for animal welfare and for doping control within the horseracing industry where drug-specific metabolites are tested for on race days. Recently the first recombinant equine CYPs have been produced, allowing specific data on equine P450 activity to be gathered for the first time. During the current study,46 full-length P450 sequences were identified from the equine genome. RT- PCR analysis was then carried out on equine liver in order to detect hepatic expression of P450s across various families. After this, cold-induction (pCold) E. coli were used for production of recombinant P450 proteins for subsquent functional testing. Four recombinant equine P450s were successfully expressed (CYP1A1, CYP2A13, CYP2C92 and CYP2D50). Due to being the isoforms most likely to be involved in drug metabolism, rCYP2D50 and rCYP2C92 were selected to be screened against ten of the most commonly used horse drugs to identify potential substrates. rCYP2C92 appeared to metabolise all four NSAIDs tested (flunixin, ketoprofen, phenylbutazone and diclofenac), however presence of the known hydroxylated metabolites of diclofenac and phenylbutazone (4-hydroxydiclofenac and oxyphenbutazone, respectively) could not be confirmed despite being present within equine liver microsome and human recombinant CYP2C9 samples. -

Differential Expression of Prostaglandin I2 Synthase Associated with Arachidonic Acid Pathway in the Oral Squamous Cell Carcinoma
Hindawi Journal of Oncology Volume 2018, Article ID 6301980, 13 pages https://doi.org/10.1155/2018/6301980 Research Article Differential Expression of Prostaglandin I2 Synthase Associated with Arachidonic Acid Pathway in the Oral Squamous Cell Carcinoma Anelise Russo ,1 Patr-cia M. Biselli-Chicote ,1 Rosa S. Kawasaki-Oyama,1 Márcia M. U. Castanhole-Nunes,1 José V. Maniglia,2 Dal-sio de Santi Neto,3 Érika C. Pavarino ,1 and Eny M. Goloni-Bertollo 1 1 Department of Molecular Biology: Biological and Genetics and Molecular Biology Research Unit – UPGEM, Sao˜ Jose´ do Rio Preto Medical School – FAMERP, Sao˜ Jose´ do Rio Preto, SP 15090-000, Brazil 2Department of Otorhinolaryngology and Head and Neck Surgery, FAMERP, Sao˜ Jose´ do Rio Preto, SP 15090-000, Brazil 3Department of Pathology, FAMERP, Sao˜ Jose´ do Rio Preto, SP 15090-000, Brazil Correspondence should be addressed to Anelise Russo; [email protected] and Eny M. Goloni-Bertollo; [email protected] Received 17 July 2018; Accepted 16 October 2018; Published 8 November 2018 Academic Editor: Tomas R. Chauncey Copyright © 2018 Anelise Russo et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Introduction. Diferential expression of genes encoding cytochrome P450 (CYP) and other oxygenases enzymes involved in biotransformation mechanisms of endogenous and exogenous compounds can lead to oral tumor development. Objective.We aimed to identify the expression profle of these genes, searching for susceptibility biomarkers in oral squamous cell carcinoma. -

Multi-Gene Next-Generation Sequencing for Molecular Diagnosis of Autosomal Recessive Congenital Ichthyosis: a Genotype-Phenotype Study of Four Italian Patients
diagnostics Article Multi-Gene Next-Generation Sequencing for Molecular Diagnosis of Autosomal Recessive Congenital Ichthyosis: A Genotype-Phenotype Study of Four Italian Patients Tiziana Fioretti 1, Luigi Auricchio 2, Angelo Piccirillo 3, Giuseppina Vitiello 4 , Adelaide Ambrosio 5, Fabio Cattaneo 5 , Rosario Ammendola 5 and Gabriella Esposito 1,5,* 1 CEINGE—Advanced Biotechnologies s.c. a r.l., Via Gaetano Salvatore, 80145 Naples, Italy; fi[email protected] 2 Department of Clinical Medicine and Surgery, University of Naples Federico II, Via S. Pansini, 5, 80131 Naples, Italy; [email protected] 3 San Carlo Hospital, Operating Unit of Dermatology, 85100 Potenza, Italy; [email protected] 4 Complex Operating Unit of Medical Genetics, University Hospital Federico II, Via S. Pansini, 5, 80131 Naples, Italy; [email protected] 5 Department of Molecular Medicine and Medical Biotechnologies, University of Naples Federico II, Via S. Pansini, 5, 80131 Naples, Italy; [email protected] (A.A.); [email protected] (F.C.); [email protected] (R.A.) * Correspondence: [email protected]; Tel.: +39-081-7463146 Received: 28 October 2020; Accepted: 21 November 2020; Published: 24 November 2020 Abstract: Autosomal recessive congenital ichthyoses (ARCI) are rare genodermatosis disorders characterized by phenotypic and genetic heterogeneity. At least fourteen genes so far have been related to ARCI; however, despite genetic heterogeneity, phenotypes associated with mutation of different ARCI genes may overlap, thereby making difficult their clinical and molecular classification. In addition, molecular tests for diagnosis of such an extremely rare heterogeneous inherited disease are not easily available in clinical settings. In the attempt of identifying the genetic cause of the disease in four Italian patients with ARCI, we performed next-generation sequencing (NGS) analysis targeting 4811 genes that have been previously linked to human genetic diseases; we focused our analysis on the 13 known ARCI genes comprised in the panel. -

Vitamin D Signaling Pathway and Breast Cancer
VITAMIN D SIGNALING PATHWAY AND BREAST CANCER Doctoral Thesis by Lei Sheng Vitamin D Signaling Pathway and Breast Cancer Lei Sheng M.Med., B.Med. This thesis is submitted in fulfilment of the requirements for the Doctor of Philosophy Adelaide Medical School The University of Adelaide Adelaide SA, Australia March 2017 Table of Contents Overview i Publications v Acknowledgement vii Declaration ix CHAPTER I 1 INTRODUCTION: VITAMIN D SIGNALING PATHWAY AND BREAST CANCER 1 1.1 Introduction 2 1.2 Vitamin D metabolism 2 1.2.1 The endocrine paradigm of vitamin D metabolism 2 1.2.2 The paracrine/autocrine paradigm of vitamin D metabolism 4 1.2.3 The paracrine/autocrine paradigm of vitamin D metabolism in the breast 5 1.3 Biological function of Vitamin D 6 1.3.1 The effect of vitamin D in bone 7 1.3.2 The effect of vitamin D in the murine mammary gland 8 1.3.3 The effect of vitamin D in cancer, particularly in breast cancer 10 1.4 Vitamin D and breast cancer risk 18 1.5 Vitamin D and the clinical outcome of breast cancer 20 1.6 Target genes of VDR signaling pathway 21 1.7 Conclusion 23 1.8 References 29 CHAPTER II 47 THE EFFECT OF VITAMIN D SUPPLEMENTATION ON THE RISK OF BREAST CANCER: A TRIAL SEQUENTIAL META-ANALYSIS 47 2.1 Prelude 48 2.2 Abstract 49 2.3 Introduction 51 2.4 Methods 53 2.4.1 Search strategy and eligibility criteria 53 2.4.2 Data collection 53 2.4.3 Data analysis 53 2.4.4 Trial sequential analysis 54 2.5 Results 55 2.5.1 Results of database search 55 2.5.2 Quality assessment of included trials 55 2.5.3 Effects of intervention 56 -

Author Pre-Print British Journal of Dermatology Epidermal Barrier
Author pre‐print British Journal of Dermatology Epidermal barrier impairment associated with CYP4F22 mutations in autosomal recessive congenital ichthyosis R. Gruber1,2,*, G. Rainer3,4*, A. Weiss5, A. Udvardi3,5, H. Thiele6, K.M. Eckl1, R. Schupart1, P. Nürnberg6, J. Zschocke1, M. Schmuth2, B. Volc‐Platzer3,4, H.C. Hennies1,2,6,7 1Division of Human Genetics, Medical University of Innsbruck, Innsbruck, Austria 2Department of Dermatology, Venereology and Allergology, Medical University of Innsbruck, Innsbruck, Austria 3Department of Dermatology, Donauspital Vienna, Vienna, Austria 4Karl Landsteiner Institute for Pediatric Dermatology, Vienna, Austria 5Department of Paediatrics, Wilhelminen Hospital, Vienna, Austria 6Cologne Center for Genomics, University of Cologne, Cologne, Germany 7Department of Biological Sciences, University of Huddersfield, Huddersfield, UK * These two authors contributed equally to this work Running head: Barrier impairment in ARCI with CYP4F22 mutations Correspondence: PD Dr. Hans Christian Hennies Dept. of Biological Sciences, University of Huddersfield, Queensgate, Huddersfield HD1 3DH, UK Phone: +44‐1484‐473014 Fax: +44‐1484‐472182 E‐mail: [email protected] 1368 words, 3 figures, 1 supplemental figure, and supplemental methods Conflicts of interest: None declared Funding sources: Austrian National Bank, German Federal Ministry for Education and Research, Köln Fortune Program of the Faculty of Medicine, University of Cologne What´s already known about this topic? ‐ Autosomal recessive congenital ichthyosis (ARCI) caused by CYP4F22 mutations is very rare. ‐ There is evidence that CyP4F22 plays a role in the 12(R)‐lipoxygenase pathway and therefore is important for skin barrier function. What does this study add? ‐ We report a novel homozygous splice site mutation c.549+5G>C in CYP4F22 in two sisters with ARCI, presenting with and without a collodion membrane at birth.