(Lama Guanicoe) in South America
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Vegetation Map of South America
A VEGETATION MAP OF SOUTH AMERICA MAPA DE LA VEGETACIÓN DE AMÉRICA DEL SUR MAPA DA VEGETAÇÃO DA AMÉRICA DO SUL H.D.Eva E.E. de Miranda C.M. Di Bella V.Gond O.Huber M.Sgrenzaroli S.Jones A.Coutinho A.Dorado M.Guimarães C.Elvidge F.Achard A.S.Belward E.Bartholomé A.Baraldi G.De Grandi P.Vogt S.Fritz A.Hartley 2002 EUR 20159 EN A VEGETATION MAP OF SOUTH AMERICA MAPA DE LA VEGETACIÓN DE AMÉRICA DEL SUR MAPA DA VEGETAÇÃO DA AMÉRICA DO SUL H.D.Eva E.E. de Miranda C.M. Di Bella V.Gond O.Huber M.Sgrenzaroli S.Jones A.Coutinho A.Dorado M.Guimarães C.Elvidge F.Achard A.S.Belward E.Bartholomé A.Baraldi G.De Grandi P.Vogt S.Fritz A.Hartley 2002 EUR 20159 EN A Vegetation Map of South America I LEGAL NOTICE Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu.int) Cataloguing data can be found at the end of this publication Luxembourg: Office for Official Publications of the European Communities, 2002 ISBN 92-894-4449-5 © European Communities, 2002 Reproduction is authorized provided the source is acknowledged Printed in Italy II A Vegetation Map of South America A VEGETATION MAP OF SOUTH AMERICA prepared by H.D.Eva* E.E. -

PATAGONIAN STEPPE Developing a Transboundary Strategy for Conservation and Sustainable Management the Challenge
PATAGONIAN STEPPE Developing a transboundary strategy for conservation and sustainable management Landscape of the Patagonian steppe The Challenge The Patagonian steppe is a vast semi-arid to arid region located on the southern tip of the continent, mainly in southern Argentina but also in Chile. Patagonia is a relatively dry and temperate region that occupies around 800,000 square kilometres. Vegetation physiognomy and floristic composition range from semi-deserts, grass and shrub steppes to humid prairies. The two main economic activities in the Patagonian steppe are the oil industry and sheep grazing. Oil extraction is the most intensive anthropogenic disturbance, though it is restricted in extent. Overgrazing has been perceived to be the main agent of desertification in the arid and semi-arid Patagonian steppe. Overall, 35% of the rangelands have been transformed into deserts. The consequences of deterioration have been profound from both the biodiversity conservation and the socio-economic perspective. Primary productivity has reduced, along with the provision of ecosystems services such as carbon capture, hydrological regulation, decomposition, nutrient cycling and mound dynamics. Climate change is already aggravating this scenario. Formal protection of Patagonian steppe is poor. There are twenty protected areas that cover around 2,500,000 hectares (approximately 3% of the region). Urgent action is crucial to guarantee the long-term preservation of these ecosystems and their provision of environmental goods and services. An integrated -

Soil Ecoregions in Latin America 5
Chapter 1 Soil EcoregionsSoil Ecoregions in Latin Latin America America Boris Volkoff Martial Bernoux INTRODUCTION Large soil units generally reflect bioclimatic environments (the concept of zonal soils). Soil maps thus represent summary documents that integrate all environmental factors involved. The characteristics of soils represent the environmental factors that control the dynamics of soil organic matter (SOM) and determine both their accumulation and degradation. Soil maps thus rep- resent a basis for quantitative studies on the accumulation processes of soil organic carbon (SOC) in soils in different spatial scales. From this point of view, however, and in particular if one is interested in general scales (large semicontinental regions), soil maps have several disadvantages. First, most soil maps take into account the intrinsic factors of the soils, thus the end results of the formation processes, rather than the processes themselves. These processes are the factors that are directly related to envi- ronmental conditions, whereas the characteristics of the soils can be inher- ited (paleosols and paleoalterations) and might no longer be in equilibrium with the present environment. Second, soils seldom are homogenous spatial entities. The soil cover is in reality a juxtaposition of several distinct soils that might differ to various degrees (from similar to highly contrasted), and might be either genetically linked or entirely disconnected. This spatial heterogeneity reflects the con- ditions in which the soils were formed and is expressed differently accord- ing to the substrates and the topography. The heterogeneity also depends on the duration of evolution of soils and the geomorphologic history, either re- gional or local, as well as on climatic gradients, which are particularly obvi- ous in mountain areas. -

Redalyc.Bienestar Animal En Bovinos De Leche: Selección De Indicadores
RIA. Revista de Investigaciones Agropecuarias ISSN: 0325-8718 [email protected] Instituto Nacional de Tecnología Agropecuaria Argentina MARTÍNEZ, G. M.; SUÁREZ, V. H.; GHEZZI, M. D. Bienestar animal en bovinos de leche: selección de indicadores vinculados a la salud y producción RIA. Revista de Investigaciones Agropecuarias, vol. 42, núm. 2, agosto, 2016, pp. 153- 160 Instituto Nacional de Tecnología Agropecuaria Buenos Aires, Argentina Disponible en: http://www.redalyc.org/articulo.oa?id=86447075008 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Agosto 2016, Argentina 15 Bienestar animal en bovinos de leche: selección de indicadores vinculados a la salud y producción MARTÍNEZ, G. M. 1*; SUÁREZ, V. H. 2; GHEZZI, M. D. 3 RESUMEN El bienestar animal ha sido denido por la Organización Mundial de Sanidad Animal (OIE) como el término amplio que describe la manera en que los individuos se enfrentan con el ambiente y que incluye su sanidad, sus percepciones, su estado anímico y otros e fectos positivos o negativos que inuyen sobre los mecanis - mos físicos y psíquicos del animal. Durante años se tuvo como objetivo principal dentro de los programas de mejora genética en rodeos lecheros el aumento en la producción de leche por individuo; posteriormente se trabajó en compatibilizar ese incremento en el rinde con una mayor eciencia en la conversión alimenticia. A lo largo de este período todo el sistema productivo se fue transformando de manera tal de ofrecerle a esos animales de alto mérito genético el ambiente necesario para que consiguiesen expresar su potencial. -

Prospects for Rewilding with Camelids
Journal of Arid Environments 130 (2016) 54e61 Contents lists available at ScienceDirect Journal of Arid Environments journal homepage: www.elsevier.com/locate/jaridenv Prospects for rewilding with camelids Meredith Root-Bernstein a, b, *, Jens-Christian Svenning a a Section for Ecoinformatics & Biodiversity, Department of Bioscience, Aarhus University, Aarhus, Denmark b Institute for Ecology and Biodiversity, Santiago, Chile article info abstract Article history: The wild camelids wild Bactrian camel (Camelus ferus), guanaco (Lama guanicoe), and vicuna~ (Vicugna Received 12 August 2015 vicugna) as well as their domestic relatives llama (Lama glama), alpaca (Vicugna pacos), dromedary Received in revised form (Camelus dromedarius) and domestic Bactrian camel (Camelus bactrianus) may be good candidates for 20 November 2015 rewilding, either as proxy species for extinct camelids or other herbivores, or as reintroductions to their Accepted 23 March 2016 former ranges. Camels were among the first species recommended for Pleistocene rewilding. Camelids have been abundant and widely distributed since the mid-Cenozoic and were among the first species recommended for Pleistocene rewilding. They show a range of adaptations to dry and marginal habitats, keywords: Camelids and have been found in deserts, grasslands and savannas throughout paleohistory. Camelids have also Camel developed close relationships with pastoralist and farming cultures wherever they occur. We review the Guanaco evolutionary and paleoecological history of extinct and extant camelids, and then discuss their potential Llama ecological roles within rewilding projects for deserts, grasslands and savannas. The functional ecosystem Rewilding ecology of camelids has not been well researched, and we highlight functions that camelids are likely to Vicuna~ have, but which require further study. -

Giant Camels from the Cenozoic of North America SERIES PUBLICATIONS of the SMITHSONIAN INSTITUTION
Giant Camels from the Cenozoic of North America SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the H^arine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that refXJrt the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the worid. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. Press requirements for manuscript and art preparation are outlined on the inside back cover. -
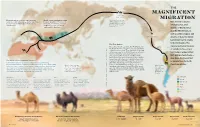
Magnificent Migration
THE MAGNIFICENT Land bridge 8 MY–14,500 Y MIGRATION Domestication of camels began between World camel population today About 6 million years ago, 3,000 and 4,000 years ago—slightly later than is about 30 million: 27 million of camelids began to move Best known today for horses—in both the Arabian Peninsula and these are dromedaries; 3 million westward across the land Camelini western Asia. are Bactrians; and only about that connected Asia and inhabiting hot, arid North America. 1,000 are Wild Bactrians. regions of North Africa and the Middle East, as well as colder steppes and Camelid ancestors deserts of Asia, the family Camelidae had its origins in North America. The The First Camels signature physical features The earliest-known camelids, the Protylopus and Lamini E the Poebrotherium, ranged in sizes comparable to of camels today—one or modern hares to goats. They appeared roughly 40 DMOR I SK million years ago in the North American savannah. two humps, wide padded U L Over the 20 million years that followed, more U L ; feet, well-protected eyes— O than a dozen other ancestral members of the T T O family Camelidae grew, developing larger bodies, . E may have developed K longer legs and long necks to better browse high C I The World's Most Adaptable Traveler? R ; vegetation. Some, like Megacamelus, grew even I as adaptations to North Camels have adapted to some of the Earth’s most demanding taller than the woolly mammoths in their time. LEWSK (Later, in the Middle East, the Syrian camel may American winters. -

Description of a Fossil Camelid from the Pleistocene of Argentina, and a Cladistic Analysis of the Camelinae
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Description of a fossil camelid from the Pleistocene of Argentina, and a cladistic analysis of the Camelinae Lynch, Sinéad ; Sánchez-Villagra, Marcelo R ; Balcarcel, Ana Abstract: We describe a well-preserved South American Lamini partial skeleton (PIMUZ A/V 4165) from the Ensenadan ( 1.95–1.77 to 0.4 Mya) of Argentina. The specimen is comprised of a nearly complete skull and mandible with full tooth rows, multiple elements of anterior and posterior limbs, and a scapula. We tested this specimen’s phylogenetic position and hypothesized it to be more closely related to Lama guanicoe and Vicugna vicugna than to Hemiauchenia paradoxa. We formulate a hypothesis for the placement of PIMUZ A/V 4165 within Camelinae in a cladistic analysis based on craniomandibular and dental characters and propose that future systematic studies consider this specimen as representing a new species. For the first time in a morphological phylogeny, we code terminal taxa at the species levelfor the following genera: Camelops, Aepycamelus, Pleiolama, Procamelus, and Alforjas. Our results indicate a divergence between Lamini and Camelini predating the Barstovian (16 Mya). Camelops appears as monophyletic within the Camelini. Alforjas taylori falls out as a basal member of Camelinae—neither as a Lamini nor Camelini. Pleiolama is polyphyletic, with Pleiolama vera as a basal Lamini and Pleiolama mckennai in a more nested position within the Lamini. Aepycamelus and Procamelus are respectively polyphyletic and paraphyletic. Together, they are part of a group of North American Lamini from the Miocene epoch. -

A Vegetation Map of South America
A VEGETATION MAP OF SOUTH AMERICA MAPA DE LA VEGETACIÓN DE AMÉRICA DEL SUR MAPA DA VEGETAÇÃO DA AMÉRICA DO SUL H.D.Eva E.E. de Miranda C.M. Di Bella V.Gond O.Huber M.Sgrenzaroli S.Jones A.Coutinho A.Dorado M.Guimarães C.Elvidge F.Achard A.S.Belward E.Bartholomé A.Baraldi G.De Grandi P.Vogt S.Fritz A.Hartley 2002 EUR 20159 EN A VEGETATION MAP OF SOUTH AMERICA MAPA DE LA VEGETACIÓN DE AMÉRICA DEL SUR MAPA DA VEGETAÇÃO DA AMÉRICA DO SUL H.D.Eva E.E. de Miranda C.M. Di Bella V.Gond O.Huber M.Sgrenzaroli S.Jones A.Coutinho A.Dorado M.Guimarães C.Elvidge F.Achard A.S.Belward E.Bartholomé A.Baraldi G.De Grandi P.Vogt S.Fritz A.Hartley 2002 EUR 20159 EN A Vegetation Map of South America I LEGAL NOTICE Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu.int) Cataloguing data can be found at the end of this publication Luxembourg: Office for Official Publications of the European Communities, 2002 ISBN 92-894-4449-5 © European Communities, 2002 Reproduction is authorized provided the source is acknowledged Printed in Italy II A Vegetation Map of South America A VEGETATION MAP OF SOUTH AMERICA prepared by H.D.Eva* E.E. -

Grasslands of the World.Pdf
The views expressed in this publication are those of the author(s) and do not necessarily reflect the views of the Food and Agriculture Organization of the United Nations. The mention or omission of specific companies, their products or brand names does not imply any endorsement or judgement by the Food and Agriculture Organization of the United Nations. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. ISBN 92-5-105337-5 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] © FAO 2005 CONTENTS Foreword xiii Acknowledgements xv Contributors xvii Glossary of technical terms and abbreviations used in the text xviii Chapter 1 – Introduction 1 Purpose of the book 13 Structure of the book 13 Complementary information resources 16 References 17 Chapter 2 – The changing face of pastoral systems in grass-dominated ecosystems of eastern Africa 19 R.S. -

Information Resources on the South American Camelids: Llamas, Alpacas, Guanacos, and Vicunas 2004-2008
NATIONAL AGRICULTURAL LIBRARY ARCHIVED FILE Archived files are provided for reference purposes only. This file was current when produced, but is no longer maintained and may now be outdated. Content may not appear in full or in its original format. All links external to the document have been deactivated. For additional information, see http://pubs.nal.usda.gov. United States Department of Information Resources on the Agriculture Agricultural Research South American Camelids: Service National Agricultural Llamas, Alpacas, Guanacos, Library Animal Welfare Information Center and Vicunas 2004-2008 AWIC Resource Series No. 12, Revised 2009 AWIC Resource Series No. 12, Revised 2009 United States Information Resources on the Department of Agriculture South American Agricultural Research Service Camelids: Llamas, National Agricultural Alpacas, Guanacos, and Library Animal Welfare Vicunas 2004-2008 Information Center AWIC Resource Series No. 12, Revised 2009 Compiled by: Jean A. Larson, M.S. Animal Welfare Information Center National Agricultural Library U.S. Department of Agriculture Beltsville, Maryland 20705 E-mail: [email protected] Web site: http://awic.nal.usda.gov Available online: http://www.nal.usda.gov/awic/pubs/Camelids/camelids.shtml Disclaimers Te U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or a part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). -

Comparative Analysis of Camelid Mitochondrial Genomes
Journal of Genetics (2019) 98:88 © Indian Academy of Sciences https://doi.org/10.1007/s12041-019-1134-x RESEARCH ARTICLE Comparative analysis of camelid mitochondrial genomes MANEE M. MANEE1,2∗ , MANAL A. ALSHEHRI1, SARAH A. BINGHADIR1, SHAHAD H. ALDHAFER3, RIYOF M. ALSWAILEM3, ABDULMALEK T. ALGARNI1, BADR M. AL-SHOMRANI1 and MOHAMED B. AL-FAGEEH1 1National Centre for Biotechnology, King Abdulaziz City for Science and Technology, Riyadh 11442, Saudi Arabia 2Centre of Excellence for Genomics, King Abdulaziz City for Science and Technology, Riyadh 11442, Saudi Arabia 3College of Computer and Information Sciences, Imam Muhammad Ibn Saud Islamic University, Riyadh 11432, Saudi Arabia *For correspondence. E-mail: [email protected]. Received 9 April 2019; revised 26 June 2019; accepted 9 July 2019 Abstract. Camelus dromedarius has played a pivotal role in both culture and way of life in the Arabian peninsula, particularly in arid regions where other domestic animals cannot be easily domesticated. Although, the mitochondrial genomes have recently been sequenced for several camelid species, wider phylogenetic studies are yet to be performed. The features of conserved gene elements, rapid evolutionary rate, and rare recombination make the mitochondrial genome a useful molecular marker for phylogenetic studies of closely related species. Here we carried out a comparative analysis of previously sequenced mitochondrial genomes of camelids with an emphasis on C. dromedarius, revealing a number of noticeable findings. First, the arrangement of mitochondrial genes in C. dromedarius is similar to those of the other camelids. Second, multiple sequence alignment of intergenic regions shows up to 90% similarity across different kinds of camels, with dromedary camels to reach 99%.