Chapter 9 Coordination Chemistry I: Structure and Isomers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Powerful Framework for the Chemical Design of Molecular Nanomagnets Yan Duan†,1,2, Joana T
Data mining, dashboards and statistics: a powerful framework for the chemical design of molecular nanomagnets Yan Duan†,1,2, Joana T. Coutinho†,*,1,3, Lorena E. Rosaleny†,*,1, Salvador Cardona-Serra1, José J. Baldoví1, Alejandro Gaita-Ariño*,1 1 Instituto de Ciencia Molecular (ICMol), Universitat de València, C/ Catedrático José Beltrán 2, 46980 Paterna, Spain 2 Department of Chemistry and the Ilse Katz Institute for Nanoscale Science & Technology, Ben-Gurion University of the Negev, Beer Sheva 84105, Israel 3 Centre for Rapid and Sustainable Product Development, Polytechnic of Leiria, 2430-028 Marinha Grande, Portugal *e-mail: [email protected], [email protected], [email protected] Abstract Three decades of intensive research in molecular nanomagnets have brought the magnetic memory in molecules from liquid helium to liquid nitrogen temperature. The enhancement of this operational temperature relies on a wise choice of the magnetic ion and the coordination environment. However, serendipity, oversimplified theories and chemical intuition have played the main role. In order to establish a powerful framework for statistically driven chemical design, we collected chemical and physical data for lanthanide-based nanomagnets to create a catalogue of over 1400 published experiments, developed an interactive dashboard (SIMDAVIS) to visualise the dataset, and applied inferential statistical analysis to it. We found that the effective energy barrier derived from the Arrhenius equation displays an excellent correlation with the magnetic memory, and that among all chemical families studied, only terbium bis-phthalocyaninato sandwiches and dysprosium metallocenes consistently present magnetic memory up to high temperature, but that there are some promising strategies for improvement. -

Introduction to Aromaticity
Introduction to Aromaticity Historical Timeline:1 Spotlight on Benzene:2 th • Early 19 century chemists derive benzene formula (C6H6) and molecular mass (78). • Carbon to hydrogen ratio of 1:1 suggests high reactivity and instability. • However, benzene is fairly inert and fails to undergo reactions that characterize normal alkenes. - Benzene remains inert at room temperature. - Benzene is more resistant to catalytic hydrogenation than other alkenes. Possible (but wrong) benzene structures:3 Dewar benzene Prismane Fulvene 2,4- Hexadiyne - Rearranges to benzene at - Rearranges to - Undergoes catalytic - Undergoes catalytic room temperature. Faraday’s benzene. hydrogenation easily. hydrogenation easily - Lots of ring strain. - Lots of ring strain. - Lots of ring strain. 1 Timeline is computer-generated, compiled with information from pg. 594 of Bruice, Organic Chemistry, 4th Edition, Ch. 15.2, and from Chemistry 14C Thinkbook by Dr. Steven Hardinger, Version 4, p. 26 2 Chemistry 14C Thinkbook, p. 26 3 Images of Dewar benzene, prismane, fulvene, and 2,4-Hexadiyne taken from Chemistry 14C Thinkbook, p. 26. Kekulé’s solution: - “snake bites its own tail” (4) Problems with Kekulé’s solution: • If Kekulé’s structure were to have two chloride substituents replacing two hydrogen atoms, there should be a pair of 1,2-dichlorobenzene isomers: one isomer with single bonds separating the Cl atoms, and another with double bonds separating the Cl atoms. • These isomers were never isolated or detected. • Rapid equilibrium proposed, where isomers interconvert so quickly that they cannot be isolated or detected. • Regardless, Kekulé’s structure has C=C’s and normal alkene reactions are still expected. - But the unusual stability of benzene still unexplained. -

Isomer Distributions of Molecular Weight 247 and 273 Nitro-Pahs in Ambient Samples, NIST Diesel SRM, and from Radical-Initiated Chamber Reactions
Atmospheric Environment 55 (2012) 431e439 Contents lists available at SciVerse ScienceDirect Atmospheric Environment journal homepage: www.elsevier.com/locate/atmosenv Isomer distributions of molecular weight 247 and 273 nitro-PAHs in ambient samples, NIST diesel SRM, and from radical-initiated chamber reactions Kathryn Zimmermann a,1, Roger Atkinson a,1,2,3, Janet Arey a,1,2,*, Yuki Kojima b,4, Koji Inazu b,5 a Air Pollution Research Center, University of California, Riverside, CA 92521, USA b Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8502, Japan article info abstract Article history: Molecular weight (mw) 247 nitrofluoranthenes and nitropyrenes and mw 273 nitrotriphenylenes (NTPs), Received 27 December 2011 nitrobenz[a]anthracenes, and nitrochrysenes were quantified in ambient particles collected in Riverside, Received in revised form CA, Tokyo, Japan, and Mexico City, Mexico. 2-Nitrofluoranthene (2-NFL) was the most abundant nitro- 28 February 2012 polycyclic aromatic hydrocarbon (nitro-PAH) in Riverside and Mexico City, and the mw 273 nitro-PAHs Accepted 5 March 2012 were observed in lower concentrations. However, in Tokyo concentrations of 1- þ 2-NTP were more similar to that of 2-NFL. NIST SRM 1975 diesel extract standard reference material was also analyzed to Keywords: examine nitro-PAH isomer distributions, and 12-nitrobenz[a]anthracene was identified for the first time. Nitro-PAH fl Atmospheric reactions The atmospheric formation pathways of nitro-PAHs were studied from chamber reactions of uo- Ambient particles ranthene, pyrene, triphenylene, benz[a]anthracene, and chrysene with OH and NO3 radicals at room Nitrotriphenylenes temperature and atmospheric pressure, with the PAH concentrations being controlled by their vapor pressures. -

Questions & Answers for the New Chemicals Program
Note: Effective January 19, 2016, PMNs must be submitted electronically. Learn more about the new e-PMN requirements. Questions & Answers for the New Chemicals Program (Q&A) U.S. Environmental Protection Agency Office of Pollution Prevention and Toxics Washington, DC 20460 2004 -1- TABLE OF CONTENTS Page 1. GENERAL PROGRAM INFORMATION 100. General ............................................................................................................ 1-1 101. Guidance for Completion of §5 Submission Form ......................................... 1-6 102. Inventory Searches/Bona Fides ....................................................................... 1-17 103. Chemical Identification ................................................................................... 1-22 104. Nomenclature .................................................................................................. 1-26 105. Inventory Issues ................................................................................................ 1-31 106. Review Process ............................................................................................... 1-31 107. Notice of Commencement .............................................................................. 1-33 108. User Fee .......................................................................................................... 1-35 109. Consolidated Notices ...................................................................................... 1-39 110. Joint Submissions .......................................................................................... -

Electrophilic Mercuration and Thallation of Benzene and Substituted Benzenes in Trifluoroacetic Acid Solution* (Electrophilic Substitution/Selectivity) GEORGE A
Proc. Natl. Acad. Sci. USA Vol. 74, No. 10, pp. 4121-4125, October 1977 Chemistry Electrophilic mercuration and thallation of benzene and substituted benzenes in trifluoroacetic acid solution* (electrophilic substitution/selectivity) GEORGE A. OLAH, IWAO HASHIMOTOt, AND HENRY C. LINtt Institute of Hydrocarbon Chemistry, Department of Chemistry, University of Southern California, Los Angeles, California 90007; and the Department of Chemistry, Case Western Reserve University, Cleveland, Ohio 44101 Contributed by George A. Olah, July 18, 1977 ABSTRACT The mercuration and thallation of benzene and iments at 250 and quenched the mixtures after 5 min, whereas substituted benzenes was studied with mercuric and thallic Brown and Nelson carried out the reaction for 6.5 hr. In sub- trifluoroacetate, respectively, in trifluoroacetic acid. With the in view of the importance of both the mechanistic shortest reaction time (1 sec) at 00, the relative rate of mercu- sequent work, ration of toluene compared to that of benzene was 17.5, with and practical implications of the orientation-rate correlation, the isomer distribution in toluene of: ortho, 17.4%; meta, 5.9%; Brown and McGary (7), carried out a more detailed study of and para, 76.7%. The isomer distribution in toluene varied with the mercuration reaction. They concluded: "A redetermination the reaction time, significantly more at 25° than at 00. The of the isomer distributions and relative rates indicates excellent competitive thallation of benzene and toluene with thallic tri- agreement with the linear relationship of orientation and rel- fluoroacetate in trifluoroacetic acid at 150 showed the relative ative however, that the isomer distri- rate, toluene/benzene, to be 33, with the isomer distribution in rate." They recognized, toluene of: ortho, 9.5%; meta, 5.5%; and para, 85.0%. -

Coordination Chemistry (See Chapter 20, H&S 3Rd Ed.)
Coordination Chemistry (see Chapter 20, H&S 3rd Ed.) Transition metal elements, particularly ions, have a strong tendency to form compounds with Lewis bases. Why? Lewis base: electron pair donor egs. H2O, NH3 n+ Lewis acid: electron pair acceptor egs. M , BH3 ‘low lying’ empty orbitals: lower in energy than HOMO of Lewis case Lewis acidity enhanced by positive charge so TM ions are good Lewis bases 2+ 3+ familiar examples: AlCl3, BF3 from main group and M , M in TM series Type of bonding in these compounds? ‘coordinative’ or ‘dative’ bonds to form Lewis base ‘adducts’ L→M TM-Lewis base ‘adducts’ are often referred to as (i) TM ‘complexes’ OR (ii) coordination complexes NB: still polar covalent bonds – this just defines where the pair of electrons came from Some more definitions: ligand: molecule or ion that can act as a Lewis base to a metal - egs. Cl , PR3, ROR (R = generic term for an alkyl) donor atoms: atoms that are in direct contact with the metal ion O in R2O→M denticity: number of donor atoms per ligand; range from 1 to >6 unidentate bidentate tridentate O H O H2N NH2 2 O O water en diglyme N O O O N N O O N porphin 15-C-5 tetradentate pentadentate chelate: multidentate ligands forms rings; M tend to be very stable, OO especially 5 and 6 member chelate rings acac ‘bite’ or ‘bite angle’: a measure of the span or size of a chelate ligand; depends on M-L distance M OO acac coordination complex MLn generic terms commonly used: L neutral Lewis base (electron pair donor) X anionic donor (eg. -

Cis-Trans Isomerism 2
Isomerism Isomerism • These compounds possess the same molecular formula but differ from each other in physical or chemical properties, and are called Isomers and the phenomenon is termed Isomerism • Since isomers have the same molecular formula, the difference in their properties must be due to different modes of combination or arrangement of atoms within the molecule. There are two main types of isomerism • (i) Structural Isomerism and (ii) Stereoisomerism. Structural Isomerism • When the isomerism is simply due to difference in the arrangement of atoms within the molecule without any reference to space, the phenomenon is termed Structural Isomerism. In other words, the structural isomers are compounds that have the same molecular formula but different structural formulas. Types of Structural Isomerism 1. Chain Isomerism 2. Position Isomerism 3. Functional Isomerism 4. Metamersim 5. Tautomerism Stereoisomerism • When isomerism is caused by the different arrangements of atoms or groups in space, the phenomenon is called Stereoisomerism (Greek, Stereos = occupying space). • The stereoisomers have the same structural formulas but differ in arrangement of atoms in space. In other words, stereoisomerism is exhibited by such compounds which have identical molecular structure but different configurations. Configuration refers to the 3-dimentional arrangement of atoms that characterizes a particular compound. Types of Stereoisomerism Stereoisomerism is of two types 1. Geometrical or Cis-Trans Isomerism 2. Optical Isomerism Structural Isomerism Chain Isomerism This type of isomerism arises from she difference in the structure of carbon chain which forms the nucleus of the molecule. It is, therefore, named as Chain or Nuclear Isomerism. Chain isomers have the same molecular formula but differ in the order in which the carbon atoms are bonded to each other For example, there are known two butanes which have the same molecular formula (C4H10) but differ in the structure of the carbon chains in their molecules. -
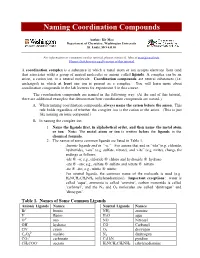
Naming Coordination Compounds
Naming Coordination Compounds Author: Kit Mao Department of Chemistry, Washington University St. Louis, MO 63130 For information or comments on this tutorial, please contact K. Mao at [email protected]. Please click here for a pdf version of this tutorial. A coordination complex is a substance in which a metal atom or ion accepts electrons from (and thus associates with) a group of neutral molecules or anions called ligands. A complex can be an anion, a cation ion, or a neutral molecule. Coordination compounds are neutral substances (i.e. uncharged) in which at least one ion is present as a complex. You will learn more about coordination compounds in the lab lectures for experiment 5 in this course. The coordination compounds are named in the following way. (At the end of this tutorial, there are additional examples that demonstrate how coordination compounds are named.) A. When naming coordination compounds, always name the cation before the anion. This rule holds regardless of whether the complex ion is the cation or the anion. (This is just like naming an ionic compound.) B. In naming the complex ion: 1. Name the ligands first, in alphabetical order, and then name the metal atom or ion. Note: The metal atom or ion is written before the ligands in the chemical formula. 2. The names of some common ligands are listed in Table 1. · Anionic ligands end in “-o.” For anions that end in “-ide”(e.g. chloride, hydroxide), “-ate” (e.g. sulfate, nitrate), and “-ite” (e.g. nirite), change the endings as follows: -ide ® -o; e.g., chloride ® chloro and hydroxide ® hydroxo -ate ® -ato; e.g., sulfate ® sulfato and nitrate ® nitrato -ite ® -ito; e.g., nitrite ® nitrito · For neutral ligands, the common name of the molecule is used (e.g. -

Alkenes and Alkynes
02/21/2019 CHAPTER FOUR Alkenes and Alkynes H N O I Cl C O C O Cl F3C C Cl C Cl Efavirenz Haloprogin (antiviral, AIDS therapeutic) (antifungal, antiseptic) Chapter 4 Table of Content * Unsaturated Hydrocarbons * Introduction and hybridization * Alkenes and Alkynes * Benzene and Phenyl groups * Structure of Alkenes, cis‐trans Isomerism * Nomenclature of Alkenes and Alkynes * Configuration cis/trans, and cis/trans Isomerism * Configuration E/Z * Physical Properties of Hydrocarbons * Acid‐Base Reactions of Hydrocarbons * pka and Hybridizations 1 02/21/2019 Unsaturated Hydrocarbons • Unsaturated Hydrocarbon: A hydrocarbon that contains one or more carbon‐carbon double or triple bonds or benzene‐like rings. – Alkene: contains a carbon‐carbon double bond and has the general formula CnH2n. – Alkyne: contains a carbon‐carbon triple bond and has the general formula CnH2n‐2. Introduction Alkenes ● Hydrocarbons containing C=C ● Old name: olefins • Steroids • Hormones • Biochemical regulators 2 02/21/2019 • Alkynes – Hydrocarbons containing C≡C – Common name: acetylenes Unsaturated Hydrocarbons • Arene: benzene and its derivatives (Ch 9) 3 02/21/2019 Benzene and Phenyl Groups • We do not study benzene and its derivatives until Chapter 9. – However, we show structural formulas of compounds containing a phenyl group before that time. – The phenyl group is not reactive under any of the conditions we describe in chapters 5‐8. Structure of Alkenes • The two carbon atoms of a double bond and the four atoms bonded to them lie in a plane, with bond angles of approximately 120°. 4 02/21/2019 Structure of Alkenes • Figure 4.1 According to the orbital overlap model, a double bond consists of one bond formed by overlap of sp2 hybrid orbitals and one bond formed by overlap of parallel 2p orbitals. -

Bridging Ligands
Chapter 9 Coordination Chemistry I: Structure and Isomers 9-1 History 9-2 Nomenclature 9-3 Isomerism 9-4 Coordination Numbers and Structure History What is coordination compound? Coordniantion compounds include compound composed of a metal atom or ion and one or more ligands that formally donate electrons to the metal. More specifically, a transition metal surrounded by neutral molecules or anions with a definite geometry. What is ligand? Ligand can be a atom, ion, and molecules. History Prussian blue (German: Preußischblau or Berliner Blau, in English Berlin blue) is a dark blueWhatpigment is coordinationused in paints and compound? formerly in blueprints. Prussian blue wasCoordination discovered complexesby accident were by pai knownnter Heinrich - although Diesbach not understood in Berlin in 1704-5,in which any sense is why - it since is also the known beginning as Berlin of chemistry, blue. (Diesbach e.g. Prussian was attempting to createblue a paint, Aureolin with ,aand red copperhue.) It vitriolhas several. different chemical names, these being iron(III) ferrocyanide, ferric ferrocyanide, iron(III) hexacyanoferrate(II), and ferricThe hexacyanoferrate. key breakthrough Commonly occurred when and c Alfredonveniently Werner it isproposed, simply called "PB. inter alia, that Co(III) bears six ligands in an octahedral geometry. Aureolin (sometimes called Cobalt Yellow) is a pigment used in oil and watercolor painting. Its color index name is PY40 (40th entry on list of yellow pigments). It was first made in 1851 and its chemical composition is potassium cobaltinitrite. Copper(II) sulfate ("sulphate" in most Commonwealth nations) is the chemical compound with the formula CuSO4. This salt exists as a series of compounds that differ in their degree of hydration. -

Enantioselective and Synergistic Herbicidal Activities of Common Amino Acids Against Amaranthus Tricolor and Echinochloa Crus-Galli
molecules Article Enantioselective and Synergistic Herbicidal Activities of Common Amino Acids Against Amaranthus tricolor and Echinochloa crus-galli Nawasit Chotsaeng 1,2,* , Chamroon Laosinwattana 3 and Patchanee Charoenying 1 1 Department of Chemistry, School of Science, King Mongkut’s Institute of Technology Ladkrabang, Bangkok 10520, Thailand; [email protected] 2 Integrated Applied Chemistry Research Unit, School of Science, King Mongkut’s Institute of Technology Ladkrabang, Bangkok 10520, Thailand 3 Department of Plant Production Technology, School of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang, Bangkok 10520, Thailand; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +66-2329-8400 (ext. 6228); Fax: +66-2329-8428 Abstract: Amino acids have a wide range of biological activities, which usually rely on the stereoiso- mer presented. In this study, glycine and 21 common α-amino acids were investigated for their herbicidal property against Chinese amaranth (Amaranthus tricolor L.) and barnyard grass (Echinochloa crus-galli (L.) Beauv.). Both D- and L-isomers, as well as a racemic mixture, were tested and found that most compounds barely inhibited germination but moderately suppressed seedling growth. Various ratios of D:L-mixture were studied and synergy between enantiomers was found. For Chinese amaranth, the most toxic D:L-mixtures were at 3:7 (for glutamine), 8:2 (for methionine), and 5:5 (for tryptophan). For barnyard grass, rac-glutamine was more toxic than the pure forms; Citation: Chotsaeng, N.; however, D-tryptophan exhibited greater activity than racemate and L-isomer, indicating the sign Laosinwattana, C.; Charoenying, P. -

Complexation
FACULTY OF PHARMACEUTICAL SCIENCES, RAMAUNIVERSITY, KANPUR B.PHARM 3rd SEM PHYSICAL PHARMACEUTICS-I BP302T MR. PEEYUSH Assistant professor Rama university, kanpur Complexation Overview Classification Introduction Metal ion complexes Organic Complexes Inclusion Complexes Methods of Analysis Method of Continuous Variation PH Titration Distribution Method Solubility Method Spectroscopy Learning Objectives 1. Define the three classes of complexes with pharmaceutically relevant examples. 2. Describe chelates, their physically properties, and what differentiates them from organic molecular complexes. 3. Describe the types of forces that hold together organic molecular complexes with examples. 4. Describe the forces in polymer–drug complexes used for drug delivery. 5. Discuss the pharmaceutical applications of cyclodextrins. 6. Describe the methods of analysis of complexes and determine their stoichiometric ratios and stability constants. Classification Introduction Metal ion complexes Organic Complexes Inclusion Complexes INTRODUCTION Complexes are compounds that result from donor–acceptor mechanisms between two or more chemical species. Complexes can be divided broadly into three classes depending the type of the acceptor substance: 1. Metal ion complexes 2. Organic molecular complexes 3. Inclusion complexes Intermolecular forces involved in the formation of complexes: 1. Van der Waals forces. 2. Hydrogen bonds (important in molecular complexes). 3. Coordinate covalence (important in metal complexes). 4. Charge transfer. 5. Hydrophobic interaction. Introduction Types of Complexes Metal Ion Complexes A. Inorganic type B. Chelates C. Olefin type D. Aromatic type II. Organic Molecular Complexes A. Quinhydrone type B. Picric acid type C. Caffeine and other drug complexes D. Polymer type III. Inclusion Compounds A. Channel lattice type B. Layer type C. Clathrates D. Monomolecular type E.