The Role of the Orbitofrontal Cortex in Regulation of Interpersonal Space
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -
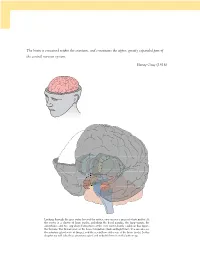
The Brain Is Contained Within the Cranium, and Constitutes the Upper, Greatly Expanded Part of the Central Nervous System
The brain is contained within the cranium, and constitutes the upper, greatly expanded part of the central nervous system. Henry Gray (1918) Looking through the gray outer layer of the cortex, you can see a mass of white matter. At the center is a cluster of large nuclei, including the basal ganglia, the hippocampi, the amygdalae, and two egg-shaped structures at the very center, barely visible in this figure, the thalami. The thalami rest on the lower brainstem (dark and light blue). You can also see the pituitary gland in front (beige), and the cerebellum at the rear of the brain (pink). In this chapter we will take these structures apart and re-build them from the bottom up. 09_P375070_Ch05.indd 126 1/29/2010 4:08:25 AM CHAPTER 5 The brain OUTLINE 1.0 Introduction 127 3.2 Output and input: the front-back division 143 1.1 The nervous system 128 3.3 The major lobes: visible and hidden 145 1.2 The geography of the brain 129 3.4 The massive interconnectivity of the cortex and thalamus 149 2.0 Growing a brain from the bottom up 133 3.5 The satellites of the subcortex 151 2.1 Evolution and personal history are expressed in the brain 133 4.0 Summary 153 2.2 Building a brain from bottom to top 134 5.0 Chapter review 153 3.0 From ‘ where ’ to ‘ what ’ : the functional 5.1 Study questions 153 roles of brain regions 136 5.2 Drawing exercises 153 3.1 The cerebral hemispheres: the left-right division 136 1.0 INTRODUCTION found. -

Diverging Patterns of Atrophy and Hypometabolism in Syndromic
Simultaneous PET-MRI studies of the concordance of atrophy and hypometabolism in syndromic variants of Alzheimer’s disease and frontotemporal dementia: an extended case series *Kuven K Moodley1, *Daniela Perani2, Ludovico Minati1,3, Pasquale Anthony Della Rosa4, Frank Pennycook5, John C Dickson6, Anna Barnes6, Valeria Elisa Contarino7, Sofia Michopoulou7, Ludovico D’Incerti7, Catriona Good8, Federico Fallanca2, Emilia Giovanna Vanoli2, Peter J Ell6, and Dennis Chan1 *These authors equally contributed to the work 1Brighton and Sussex Medical School, Falmer, UK 2 Vita-Salute San Raffaele University, Nuclear Medicine Unit San Raffaele Hospital, Division of Neuroscience IRCCS San Raffaele, Milano, Italy 3Scientific Department, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano, Italy 4Institute of Molecular Bio-imaging and Physiology, National Research Council, Milano, Italy 5Open University, Milton Keynes, UK 6Institute of Nuclear Medicine, University College London, London, UK 7Neuroradiology Department, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano, Italy 8Hurstwood Park Neurosciences Centre, West Sussex, UK Corresponding Author: Dr Dennis Chan Herchel Smith Building for Brain and Mind Sciences Department of Clinical Neurosciences University of Cambridge Forvie Site, Robinson Way Cambridge CB2 0SZ Email: [email protected] Word count Abstract 278 Main Text 4969 Tables 1 Figures 6 Supplementary Figures 6 1 Abstract Aims. The primary objective was to determine the concordance of brain atrophy and hypometabolism in six syndromic variants of Alzheimer’s disease (AD) and frontotemporal dementia spectrum (FTD). A second objective was to determine the effect of image analysis methods on identification of atrophy and hypometabolism by comparing the changes observed through qualitative rating with those detected by quantitative methods. -

A Brief Anatomical Sketch of Human Ventromedial Prefrontal Cortex Jamil P
This article is a supplement referenced in Delgado, M. R., Beer, J. S., Fellows, L. K., Huettel, S. A., Platt, M. L., Quirk, G. J., & Schiller, D. (2016). Viewpoints: Dialogues on the functional role of the ventromedial prefrontal cortex. Nature Neuroscience, 19(12), 1545-1552. Brain images used in this article (vmPFC mask) are available at https://identifiers.org/neurovault.collection:5631 A Brief Anatomical Sketch of Human Ventromedial Prefrontal Cortex Jamil P. Bhanji1, David V. Smith2, Mauricio R. Delgado1 1 Department of Psychology, Rutgers University - Newark 2 Department of Psychology, Temple University The ventromedial prefrontal cortex (vmPFC) is a major focus of investigation in human neuroscience, particularly in studies of emotion, social cognition, and decision making. Although the term vmPFC is widely used, the zone is not precisely defined, and for varied reasons has proven a complicated region to study. A difficulty identifying precise boundaries for the vmPFC comes partly from varied use of the term, sometimes including and sometimes excluding ventral parts of anterior cingulate cortex and medial parts of orbitofrontal cortex. These discrepancies can arise both from the need to refer to distinct sub-regions within a larger area of prefrontal cortex, and from the spatially imprecise nature of research methods such as human neuroimaging and natural lesions. The inexactness of the term is not necessarily an impediment, although the heterogeneity of the region can impact functional interpretation. Here we briefly address research that has helped delineate sub-regions of the human vmPFC, we then discuss patterns of white matter connectivity with other regions of the brain and how they begin to inform functional roles within vmPFC. -

Brown Medical School Biomed 370 the Brain and Human Behavior
Brown Medical School Biomed 370 The Brain and Human Behavior The Brain and Human Behavior Biomed 370 A first year, second semester course sponsored by the Department of Psychiatry and Human Behavior Course Directors: Robert Boland, M.D. 455-6417 (office), 455-6497 (fax) [email protected] Stephen Salloway, M.D., M.S. 455-6403 (office), 455-6405 (fax) [email protected] Web site available through WebCT Teaching Assistants: Nancy Brim ([email protected]) Marisa Kastoff ([email protected]) Stan Pelosi ([email protected]) Grace Farris ([email protected]) Table of Contents. Overall Course Objectives.....................................................................4 Section 1. Basic Principles. ...................................................................7 Chapter 1. Limbic System Anatomy........................................................8 Chapter 2. Frontal Lobe Function And Dysfunction..............................13 Chapter 3. Clinical Neurochemistry......................................................19 Chapter 4. The Neurobiology Of Memory............................................36 Chapter 5. The Control Of Feeding Behavior .......................................46 Chapter 6. Principles Of Pharmacology................................................51 Chapter 7. Principles Of Neuroimaging.................................................55 Chapter 8. The Mental Status Examination...........................................74 Section 2. The Clinical Disorders.......................................................89 -

Functional Connectivity of the Precuneus in Unmedicated Patients with Depression
Biological Psychiatry: CNNI Archival Report Functional Connectivity of the Precuneus in Unmedicated Patients With Depression Wei Cheng, Edmund T. Rolls, Jiang Qiu, Deyu Yang, Hongtao Ruan, Dongtao Wei, Libo Zhao, Jie Meng, Peng Xie, and Jianfeng Feng ABSTRACT BACKGROUND: The precuneus has connectivity with brain systems implicated in depression. METHODS: We performed the first fully voxel-level resting-state functional connectivity (FC) neuroimaging analysis of depression of the precuneus, with 282 patients with major depressive disorder and 254 control subjects. RESULTS: In 125 unmedicated patients, voxels in the precuneus had significantly increased FC with the lateral orbitofrontal cortex, a region implicated in nonreward that is thereby implicated in depression. FC was also increased in depression between the precuneus and the dorsolateral prefrontal cortex, temporal cortex, and angular and supramarginal areas. In patients receiving medication, the FC between the lateral orbitofrontal cortex and precuneus was decreased back toward that in the control subjects. In the 254 control subjects, parcellation revealed superior anterior, superior posterior, and inferior subdivisions, with the inferior subdivision having high connectivity with the posterior cingulate cortex, parahippocampal gyrus, angular gyrus, and prefrontal cortex. It was the ventral subdivision of the precuneus that had increased connectivity in depression with the lateral orbitofrontal cortex and adjoining inferior frontal gyrus. CONCLUSIONS: The findings support the theory that the system in the lateral orbitofrontal cortex implicated in the response to nonreceipt of expected rewards has increased effects on areas in which the self is represented, such as the precuneus. This may result in low self-esteem in depression. The increased connectivity of the precuneus with the prefrontal cortex short-term memory system may contribute to the rumination about low self-esteem in depression. -

Impairment of Social Perception Associated with Lesions of the Prefrontal Cortex
Article Impairment of Social Perception Associated With Lesions of the Prefrontal Cortex Linda Mah, M.D. Objective: Behavioral and social im- Results: Relative to the comparison sub- pairments have been frequently reported jects, patients whose lesions involved the Miriam C. Arnold, M.A. after damage to the prefrontal cortex in orbitofrontal cortex demonstrated im- humans. This study evaluated social per- paired social perception. Contrary to pre- Jordan Grafman, Ph.D. ception in patients with prefrontal cortex dictions, patients with lesions in the dor- lesions and compared their performance solateral prefrontal cortex also showed on a social perception task with that of deficits in using social cues to make inter- healthy volunteers. personal judgments. All patients, particu- larly those with lesions in the dorsolateral Method: Thirty-three patients with pre- prefrontal cortex, showed poorer insight frontal cortex lesions and 31 healthy into their deficits, relative to healthy volunteers were tested with the Interper- volunteers. sonal Perception Task. In this task, sub- Conclusions: These findings of deficits in jects viewed videotaped social interac- social perception after damage to the or- tions and relied primarily on nonverbal bitofrontal cortex extend previous clinical cues to make interpersonal judgments, and experimental evidence of damage- such as determining the degree of inti- related impairment in other aspects of so- macy between two persons depicted in cial cognition, such as the ability to accu- the videotaped scene. Patients with pre- rately evaluate emotional facial expres- frontal cortex lesions were classified ac- sions. In addition, the results suggest that cording to lesion involvement of specific the dorsolateral prefrontal cortex is re- regions, including the orbitofrontal cor- cruited when inferences about social in- tex, dorsolateral prefrontal cortex, and teractions are made on the basis of non- anterior cingulate cortex. -

SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This
12/19/2018 11:00 AM FOUNDATIONAL LEARNING SYSTEM 092892-181219 © Johnson & Johnson Servicesv Inc. 2018 All rights reserved. 1 SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This module will strengthen your understanding of basic neuroanatomy, neurovasculature, and functional roles of specific brain regions. 1 12/19/2018 11:00 AM Lesson 1: Introduction to the Brain The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. In this lesson, we’ll discuss the principle brain regions, layers of the brain, and lobes of the brain, as well as common terms used to orient neuroanatomical discussions. 2 SAY: The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. (Purves 2012/p717/para1) In this lesson, we’ll explore these organizational layers by discussing the principle brain regions, layers of the brain, and lobes of the brain. We’ll also discuss the terms used by scientists and healthcare providers to orient neuroanatomical discussions. 2 12/19/2018 11:00 AM Lesson 1: Learning Objectives • Define terms used to specify neuroanatomical locations • Recall the 4 principle regions of the brain • Identify the 3 layers of the brain and their relative location • Match each of the 4 lobes of the brain with their respective functions 3 SAY: Please take a moment to review the learning objectives for this lesson. 3 12/19/2018 11:00 AM Directional Terms Used in Anatomy 4 SAY: Specific directional terms are used when specifying the location of a structure or area of the brain. -

Increased Functional Connectivity of the Posterior Cingulate Cortex with the Lateral Orbitofrontal Cortex in Depression Wei Cheng1, Edmund T
Cheng et al. Translational Psychiatry (2018) 8:90 DOI 10.1038/s41398-018-0139-1 Translational Psychiatry ARTICLE Open Access Increased functional connectivity of the posterior cingulate cortex with the lateral orbitofrontal cortex in depression Wei Cheng1, Edmund T. Rolls2,3,JiangQiu4,5, Xiongfei Xie6,DongtaoWei5, Chu-Chung Huang7,AlbertC.Yang8, Shih-Jen Tsai 8,QiLi9,JieMeng5, Ching-Po Lin 1,7,10,PengXie9,11,12 and Jianfeng Feng1,2,13 Abstract To analyze the functioning of the posterior cingulate cortex (PCC) in depression, we performed the first fully voxel- level resting state functional-connectivity neuroimaging analysis of depression of the PCC, with 336 patients with major depressive disorder and 350 controls. Voxels in the PCC had significantly increased functional connectivity with the lateral orbitofrontal cortex, a region implicated in non-reward and which is thereby implicated in depression. In patients receiving medication, the functional connectivity between the lateral orbitofrontal cortex and PCC was decreased back towards that in the controls. In the 350 controls, it was shown that the PCC has high functional connectivity with the parahippocampal regions which are involved in memory. The findings support the theory that the non-reward system in the lateral orbitofrontal cortex has increased effects on memory systems, which contribute to the rumination about sad memories and events in depression. These new findings provide evidence that a key target to ameliorate depression is the lateral orbitofrontal cortex. 1234567890():,; 1234567890():,; Introduction pathophysiology of major depression and it appears to be Depression characterized by persistently sad or related to rumination and depression severity in MDD6. -

Tutorial: Memory and Memory Problems
Tutorial: Memory and Memory Problems WHAT IS MEMORY? Memory is one of the central components of human cognition, including the ability to take in information, process it, store it, and subsequently retrieve it when necessary. Thus the core processes of memory are encoding, storage, and retrieval: Encoding: Processing information, organizing it, and marking it for storage Storage: Holding information over time in what is ideally an organized storage system Retrieval: Calling stored information to consciousness Following TBI, both encoding and retrieval can be significantly impaired. However, storage (i.e., keeping information in storage after it has been effectively processed) is often relatively spared. Therefore, if information can be effectively processed and encoded, it is more likely to be retained, even though it may be difficult to retrieve. Video Illustration of Types of Retrieval Memory Processes and Systems Authorities on memory typically explain human memory by drawing a variety of distinctions among different types or aspects of memory. Understanding many of these distinctions is important for staff and family members working with students with memory and learning impairments. Voluntary and involuntary memory: Encoding: Encoding of information for storage in long-term memory can be either involuntary (incidental, implicit) or voluntary (effortful, deliberate, strategic). Involuntary encoding occurs when the goal of the activity is something other than memory or learning, and memory occurs as a bi-product. For example, a young student may remember the names of geometric figures not by trying to memorize them, but rather because he was involved in an art project in which it was important to process the names of the geometric figures in order to complete the project. -

Memory and the Brain
CHAPTER 2 Memory and the Brain T he word brain really means different things to different people. In everyday usage, the word brain is nearly synonymous with the word mind. We say that we have something “in our brain that we cannot get out,” meaning we have been thinking about something. You call someone a “brain” if you think that his or her intelligence is that person’s chief char - acteristic. However, underlying this metaphor is the certainty that the brain is the biologi - cal organ responsible for thinking, memory, reasoning, and language. In this chapter, we will explore the science of how the brain produces memory. For a neurosurgeon, the brain is a mass of soft tissue inside the head that has to be han - dled very carefully when damaged. The brain itself has no pain receptors, so neurosurgeons are less concerned about anesthesia than other doctors. However, the brain is surrounded and infused with millions of blood vessels, so surgeons must be very careful when probing around the brain, lest they accidentally induce a hemorrhage. Neurosurgeons understand the critical nature of the human brain for what it is to be human, yet for a surgeon, its iden - tity is a biological tissue. For a cognitive neuroscientist, the brain is a complex assortment of separate areas and regions, each of which has its own unique function. For example, the frontal regions are for planning, thinking, and monitoring, while the back of the brain processes vision. Viewed this way, the brain is not really one organ but many dozens of distinct regions each with its own appearance, its own micro-anatomy, and its own function. -

Medial Orbitofrontal Cortex, Dorsolateral Prefrontal Cortex, and Hippocampus Differentially Represent the Event Saliency
Medial Orbitofrontal Cortex, Dorsolateral Prefrontal Cortex, and Hippocampus Differentially Represent the Event Saliency Anna Jafarpour1,2, Sandon Griffin1, Jack J. Lin3, and Robert T. Knight1 Abstract ■ Two primary functions attributed to the hippocampus and watched a movie that had varying saliency of a novel or an prefrontal cortex (PFC) network are retaining the temporal anticipated flow of salient events. Using intracranial electro- and spatial associations of events and detecting deviant events. encephalography from 10 patients, we observed that high-frequency It is unclear, however, how these two functions converge into activity (50–150 Hz) in the hippocampus, dorsolateral PFC, one mechanism. Here, we tested whether increased activity and medial OFC tracked event saliency. We also observed that with perceiving salient events is a deviant detection signal or medial OFC activity was stronger when the salient events were contains information about the event associations by reflecting anticipated than when they were novel. These results suggest the magnitude of deviance (i.e., event saliency). We also tested that dorsolateral PFC and medial OFC, as well as the hippo- how the deviant detection signal is affected by the degree of campus, signify the saliency magnitude of events, reflecting anticipation. We studied regional neural activity when people the hierarchical structure of event associations. ■ INTRODUCTION more salient new events (Figure 1C; also see Yeung, “I was waiting at home for my friend. I made some tea, Yeo, & Liu, 1996). washed the cups, and poured hot water. Then I felt The magnitude of deviance of events is referred to as everything shaking. It was an earthquake.