24-Hour Urine Comprehensive Hormone Profile Handbook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
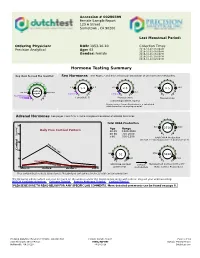
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

Association Between CYP17A1, CYP19A1, and HSD17B1 Gene Polymorphisms in Hormone Synthesis Pathway with Ovarian Cancer Risk
Association between CYP17A1, CYP19A1, and HSD17B1 Gene Polymorphisms in Hormone Synthesis pathway with Ovarian Cancer Risk Gowtham Kumar G1, Andrea Francis1, Solomon Paul1, Chirag Molia1, Manickavasagam M1, Usha Rani1, Ramya R1, and Nalini Ganesan1 1Sri Ramachandra Institute of Higher Education and Research August 27, 2020 Abstract Objective: To investigate the polymorphisms of genes in the steroidogenesis pathway to understand the etiological mechanisms to OC risk in the South Indian population Design: Case-Control Study Setting and Sample: Ovarian cancer cases (200) and healthy individuals (200) from the South Indian population. Methods: All the cases and controls were genotyped for SNPs by using allelic discrimination assay. Main outcome measures: Genetic distribution of SNPs of Steroidogenesis pathway genes in the South Indian population. Results: The observed results for rs743752, the homozygous CC genotype revealed significant association (OR; 1.68; 95%CI, 1.25-2.26; p =<0.05) and the dominant model, recessive model and additive model showed a significant association with an OR of 1.62; 95%CI, 1.09 { 2.42; p = 0.015, OR of 0.29, 95%CI, 0.14 { 0.60; p = <0.001 and OR of 1.68, 95%CI, 1.25 { 2.26); p = <0.001 respectively in cases and controls for OC risk. In rs10046, the heterozygous CT genotype (OR; 1.61; 95%CI 1.06 { 2.43; p = 0.023), the dominant (OR; 1.65; 95%CI, 1.11 { 2.45; p = 0.012) and the additive (OR; 1.46; 95%CI, 1.07 - 1.98; p = 0.015) models were found to be statistically significant. -

01 Front.Pdf
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. STUDIES TOWARDS THE DEVELOPMENT OF A MULTI PURPOSE HOME SELF-TEST KIT FOR THE DETECTION OF URINARY TETRAHYDROCORTISONE AND TESTOSTERONE METABOLITES A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Chemistry at Massey University Claire Margaret Nielsen 2003 ii Abstract The development of homogeneous enzyme immunoassays (HEIA) for testosterone glucuronide (TG) and tetrahydrocortisone glucuronide (THEG) in urine are described. The proposed test system is based on the Ovarian Monitor homogeneous immunoassay system, established by J.B Brown and L.F. Blackwell et al. 1 as a simple, laboratory accurate, monitoring device for the measurement of estrone glucuronide (E1G) and pregnanediol glucuronide (PdG) as markers of the fertile phase during a womans menstrual cycle. This information can be used readily by women to identify their cyclical periods of fertility and infertility. The major testosterone metabolite in the urine of males, testosterone p-glucuronide, was synthesised by firstly preparing the glycosyl donor a-bromosugar and conjugating this with testosterone under standard Koenigs-Knorr conditions. 1H nmr studies confirmed that the synthetic steroid glucuronide had the same stereochemistry as the naturally occurring urinary testosterone glucuronide. Testosterone glucuronide and tetrahydrocortisone glucuronide conjugates of hen egg white lysozyme were prepared using the active ester coupling method in good yield. Unreacted lysozyme was successfully removed from the reaction mixture by a combination of cation exchange chromatography in 7 M urea and hydrophobic-interaction chromatography. -

Evaluation of Deficiency of 21 -Hydroxylation in Patients with Congenital Adrenal Hyperplasia 0
Arch Dis Child: first published as 10.1136/adc.43.230.410 on 1 August 1968. Downloaded from Arch. Dis. Childh., 1968, 43, 410. Evaluation of Deficiency of 21 -hydroxylation in Patients with Congenital Adrenal Hyperplasia 0. M. GALAL, B. T. RUDD, and N. M. DRAYER From the Institute of Child Health, University of Birmingham Congenital adrenal hyperplasia can manifest therapy in these 13 children started within the first 3 itself in a variety of clinical and biochemical weeks of life. abnormalities (Bongiovanni and Root, 1963). The In addition to the 18 patients with congenital adrenal salt-losing tendency in some of these patients can hyperplasia, 3 children with shortness of stature and 3 with early signs of puberty were given ACTH to be due to the absence of specific enzymes: dehydro- investigate their adrenal function. None of these genases or hydroxylases (Bongiovanni and Root, patients had received steroids and all had normal free 1963; Ulick et al., 1964; Visser and Cost, 1964). cortisol and 17-hydroxycorticosteroid urinary excretion In addition to the impaired production of certain rates. The age and sex of the patients in the 3 groups steroids, antagonism by steroids or other compounds and details of the steroid therapy are listed in Table I. could, theoretically, account for the salt-losing tendency (Neher, Meystre, and Wettstein, 1959; ACTH test. Twenty-four hour urine collections Jacobs et al., 1961). were obtained before and during ACTH stimulation. The purpose ofthis study was to discover whether, ACTH gel (Organon) was given intramuscularly at a copyright. despite the continuation of steroid therapy, stimula- dose of 20 I.U. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

Hsd17b1) Inhibitor for Endometriosis
DEVELOPMENT OF HYDROXYSTEROID (17-BETA) DEHYDROGENASE TYPE 1 (HSD17B1) INHIBITOR FOR ENDOMETRIOSIS Niina Saarinen1,2, Tero Linnanen1, Jasmin Tiala1, Camilla Stjernschantz1, Leena Hirvelä1, Taija Heinosalo2, Bert Delvoux3, Andrea Romano3, Gabriele Möller4, Jerzy Adamski4, Matti Poutanen2, Pasi Koskimies1 1Forendo Pharma Ltd, Finland; 2Institute of Biomedicine, Research Centre for Integrative Physiology and Pharmacology, University of Turku, Finland; 3Department of Obstetrics and Gynaecology; GROW, School for Oncology and Developmental Biology; Maastricht University Medical Centre, The Netherlands; 4Institute of Experimental Genetics, Genome Analysis Center, Helmholtz Zentrum München, Germany BACKGROUND OBJECTIVE Local activation of estrogens in endometriosis tissue The main objective of the present work was to assess is considered important for growth of the lesions. the preclinical efficacy of the novel HSD17B1 inhibitor, Hydroxysteroid (17-beta) dehydrogenase type 1 FOR-6219 (HSD17B1) is expressed in endometriosis tissue and converts the biologically low-active estrogen, estrone (E1), to the highly active estradiol (E2), while hydroxysteroid (17-beta) dehydrogenase type 2 (HSD17B2), catalyzes the opposite reaction. In contrast to eutopic endometrium, in endometriotic lesions the HSD17B1/HSD17B2 expression ratio is increased and E2 levels are higher than those of E1 throughout the menstrual cycle. Thus, inhibition of HSD17B1 is considered as a feasible strategy for lowering local E2 production in endometriosis. MAIN RESULTS FOR-6219 inhibits human HSD17B1 Ø FOR-6219 is a potent and FOR-6219 does not trigger estrogenic fully selective inhibitor of response in immature rat uterine human HSD17B1 over growth assay HSD17B2 Ø FOR-6219 does not bind to estrogen receptor α or β, and exhibits no estrogen-like response in immature rat uterotrophic assay Ø FOR-6219 inhibits HSD17B1 in cynomolgus monkey, dog and rabbit i.e. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

Chuanxiong Rhizoma Compound on HIF-VEGF Pathway and Cerebral Ischemia-Reperfusion Injury’S Biological Network Based on Systematic Pharmacology
ORIGINAL RESEARCH published: 25 June 2021 doi: 10.3389/fphar.2021.601846 Exploring the Regulatory Mechanism of Hedysarum Multijugum Maxim.-Chuanxiong Rhizoma Compound on HIF-VEGF Pathway and Cerebral Ischemia-Reperfusion Injury’s Biological Network Based on Systematic Pharmacology Kailin Yang 1†, Liuting Zeng 1†, Anqi Ge 2†, Yi Chen 1†, Shanshan Wang 1†, Xiaofei Zhu 1,3† and Jinwen Ge 1,4* Edited by: 1 Takashi Sato, Key Laboratory of Hunan Province for Integrated Traditional Chinese and Western Medicine on Prevention and Treatment of 2 Tokyo University of Pharmacy and Life Cardio-Cerebral Diseases, Hunan University of Chinese Medicine, Changsha, China, Galactophore Department, The First 3 Sciences, Japan Hospital of Hunan University of Chinese Medicine, Changsha, China, School of Graduate, Central South University, Changsha, China, 4Shaoyang University, Shaoyang, China Reviewed by: Hui Zhao, Capital Medical University, China Background: Clinical research found that Hedysarum Multijugum Maxim.-Chuanxiong Maria Luisa Del Moral, fi University of Jaén, Spain Rhizoma Compound (HCC) has de nite curative effect on cerebral ischemic diseases, *Correspondence: such as ischemic stroke and cerebral ischemia-reperfusion injury (CIR). However, its Jinwen Ge mechanism for treating cerebral ischemia is still not fully explained. [email protected] †These authors share first authorship Methods: The traditional Chinese medicine related database were utilized to obtain the components of HCC. The Pharmmapper were used to predict HCC’s potential targets. Specialty section: The CIR genes were obtained from Genecards and OMIM and the protein-protein This article was submitted to interaction (PPI) data of HCC’s targets and IS genes were obtained from String Ethnopharmacology, a section of the journal database. -

Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes
Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes May 2013 Authors Mark Ferrey Contributors/acknowledgements The MPCA is reducing printing and mailing costs This report contains the results of a study that by using the Internet to distribute reports and characterizes the presence of unregulated information to wider audience. Visit our website contaminants in Minnesota’s lakes. The study for more information. was made possible through funding by the MPCA reports are printed on 100 percent post- Minnesota Clean Water Fund and by funding by consumer recycled content paper manufactured the U.S. Environmental Protection Agency without chlorine or chlorine derivatives. (EPA), which facilitated the sampling of lakes for this study. The Minnesota Pollution Control Agency (MPCA) thanks the following for assistance and advice in designing and carrying out this study: Steve Heiskary, Pam Anderson, Dereck Richter, Lee Engel, Amy Garcia, Will Long, Jesse Anderson, Ben Larson, and Kelly O’Hara for the long hours of sampling for this study. Cynthia Tomey, Kirsten Anderson, and Richard Grace of Axys Analytical Labs for the expert help in developing the list of analytes for this study and logistics to make it a success. Minnesota Pollution Control Agency 520 Lafayette Road North | Saint Paul, MN 55155-4194 | www.pca.state.mn.us | 651-296-6300 Toll free 800-657-3864 | TTY 651-282-5332 This report is available in alternative formats upon request, and online at www.pca.state.mn.us. Document number: tdr-g1-16 Contents Contents ........................................................................................................................................... -

Endocrinology Test List Endocrinology Test List
For Endocrinologists Endocrinology Test List Endocrinology Test List Extensive Capabilities Managing patients with endocrine disorders is complex. Having access to the right test for the right patient is key. With a legacy of expertise in endocrine laboratory diagnostics, Quest Diagnostics offers an extensive menu of laboratory tests across the spectrum of endocrine disorders. This test list highlights the extensive menu of laboratory diagnostic tests we offer, including highly specialized tests and those performed using highly specific and sensitive mass spectrometry detection. It is conveniently organized by glandular function or common endocrine disorder, making it easy for you to identify the tests you need to care for the patients you treat. Comprehensive Care Quest Diagnostics Nichols Institute has been pioneering state-of-the-art endocrine testing for over four decades. Our commitment to innovative diagnostics and our dedication to quality and service means we deliver solutions that enable you to make informed clinical decisions for comprehensive patient management. We strive to remain at the forefront of innovation in endocrine testing so you can deliver the highest level of patient care. Abbreviations and Footnotes NDM, neonatal diabetes mellitus; MODY, maturity-onset diabetes of the young; CH, congenital hyperinsulinism; MSUD, maple syrup urine disease; IHH, idiopathic hypogonadotropic hypogonadism; BBS, Bardet-Biedl syndrome; OI, osteogenesis imperfecta; PKD, polycystic kidney disease; OPPG, osteoporosis-pseudoglioma syndrome; CPHD, combined pituitary hormone deficiency; GHD, growth hormone deficiency. The tests highlighted in green are performed using highly specific and sensitive mass spectrometry detection. Panels that include a test(s) performed using mass spectrometry are highlighted in yellow. For tests highlighted in blue, refer to the Athena Diagnostics website (athenadiagnostics.com/content/test-catalog) for test information. -

Comprehensive Urinary Hormone Assessments
ENDOCRINOLOGY Complete Hormones – Analytes Comprehensive Urinary Hormone Assessments Urinary Pregesterones Urinary Glucocorticoids Urinary Androgens Urinary Estrogens Pregnanediol Cortisol, Free Testosterone Estrone Pregnanetriol Total 17-Hydroxy-corticosteroids Dehydroepiandrosterone (DHEA) Estradiol allo-Tetrahydrocortisol, a-THF Total 17-Ketosteroids Estriol Tetrahydrodeoxycortisol Androsterone 2-Hydroxyestrone Tetrahydrocortisol, THF Etiocholanolone 2-Methoxyestrone Tetrahydrocortisone, THE 11-Keto-androsterone 4-Hydroxyestrone 17-Hydroxysteriods, Total 11-Keto-etiocholanolone 4-Methoxyestrone Pregnanetriol 11-Hydroxy-androsterone 16α-Hydroxyestrone 11-Hydroxy-etiocholanolone 2-Hydroxy-estrone:16α-Hydroxyestrone ratio 2-Methoxyestrone:2-Hydroxyestrone ratio CLINICIAN INFORMATION 4-Methoxyestrone:4-Hydroxyestrone ratio ADVANCING THE CLINICAL UTILITY OF URINARY HORMONE ASSESSMENT Specimen Requirements Complete Hormones™ is Genova’s most comprehensive • 120 ml aliquot, refrigerated until shipped, urinary hormone profile, and is designed to assist with the from either First Morning Urine or 24-Hour clinical management of hormone-related symptoms. This profile Collection Why Use Complete Hormones? assesses parent hormones and their metabolites as well as key metabolic pathways, and provides insight into the contribution Hormone testing is an effective tool for assessing Related Profiles: that sex hormones may have in patients presenting with and managing patients with hormone- related symptoms. This profile supports: • Male Hormonal Health™ -

The Metabolism of Anabolic Agents in the Racing Greyhound
The Metabolism of Anabolic Agents In the Racing Greyhound A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy by Mr. Keith Robert Williams, B.Sc. July 1999 Department of Forensic Medicine & Science University of Glasgow Copyright © 1999 by Keith R. Williams. All rights reserved. No part o f this thesis may be reproduced in any forms or by any means without the written permission o f the author. I ProQuest Number: 13833925 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13833925 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 GLASGOW UNIVERSITY LIBRARY 111-X (coK To my parents for all their help, support and encouragement i Table of Contents i List of Figures V List of Tables VIII Summary IX Chapter 1: Drugs in Sport ...............................................................................................................................1 Introduction .................................................................................................................................................