The Gene Regulatory Control of Sea Urchin Gastrulation T Charles A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lake Worth Lagoon
TABLE OF CONTENTS INTRODUCTION ................................................................................ 1 PURPOSE AND SIGNIFICANCE OF THE PARK ...................................... 1 Park Significance .............................................................................. 1 PURPOSE AND SCOPE OF THE PLAN ................................................... 2 MANAGEMENT PROGRAM OVERVIEW ................................................. 7 Management Authority and Responsibility ............................................ 7 Park Management Goals .................................................................... 8 Management Coordination ................................................................. 8 Public Participation ........................................................................... 9 Other Designations ........................................................................... 9 RESOURCE MANAGEMENT COMPONENT INTRODUCTION .............................................................................. 15 RESOURCE DESCRIPTION AND ASSESSMENT ................................... 15 Natural Resources .......................................................................... 16 Topography ............................................................................... 20 Geology .................................................................................... 21 Soils ......................................................................................... 21 Minerals ................................................................................... -

Driwer in Fatal Island Accident Acquitted Charming and Old Isn't
Contract awarded Shell Museum construction to begin 4A io/U'q3 Since 1961 Still first on Sanibei and q<p7 3 Captiva islands VOL. 32, NO. 33 TUESDAY, AUGUST 17,1S 3 SECTIONS, 32 PAGES 50 CENTS Driwer in fatal island accident acquitted By Frances Adams Islander staff writer Carolyn Lamar Pickett of Sanibei was acquitted Aug. 11 on charges that she failed to yield right of way to pedestrians in a crosswalk, after an accident on Jan. 14 which killed a man in front of the Sanibei Community Center. Rhode Island resident retired Admiral John Remey Wadleigh, 77, was killed in the accident, and his wife, Rae, was severely injured. They were crossing Periwinkle Way after attending an Audubon Society presentation at the Sanibei Community Center. Judge Radford Sturgis made his ruling of not guilty after hearing testimony from witnesses Alice Kyllo and Sanibei Police Sgt. William Tomlinson, and from Pickett and her attorney. Sturgis said there was reasonable doubt, because the witnesses and the driver presented three com- pletely different versions and there simply wasn't enough evidence to convict her. Sturgis received a telephone call after the trial from Photo by Kathleen Biase another witness, islander Dr. Faye Granberry, who informed him that there had been other local witnesses, Beachin' it herself and two other police officers, and wondered why none of them had been subpoenaed. Granberry also told Little 2-year-old Meghan Rose tries to talk her mother, Mary Jane Rose, into playing some "sand games" with her. Sturgis what she had seen the night of the accident. -

Scaled Polychaetes
Scaled Polychaetes (Polynoidae) Associated with Ophiuroids and Other Invertebrates and Review of Species Referred to Malmgrenia Mclntosh and Replaced by Malmgreniella Hartman, with Descriptions of New Taxa MARIAN H. PETTIBONE I SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 538 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs'submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -
2.3 Million Needed to Fix Armory
Perfecting the presidents, A6 Thursday, February 27, 2014 WWW.APALACHTIMES.COM VOL. 128 ISSUE 44 50¢ xxxxxOut to see ARCHITECT ESTIMATE: Chili Cookoff on island Saturday More than 40 chili cooks $2.3 million needed to fi x Armory will liven up St. George Island Saturday. The event By LOIS SWOBODA at the Feb. 18 meeting, Emo laid ing support to wash out and leav- control out there, and the side- begins with an art review 653-1819 | @ ApalachTimes out two possible options for reno- ing the fl oor spongy under the walk’s in pretty bad condition,” he from 5-7 p.m. Friday at the lswoboda@starfl .com vating the historic structure, di- southwest corner. said. “We’re looking at new side- Civil Hall in the East End viding the repairs and upgrades Inside the building, there is walk all around the perimeter.” Firehouse. Cost is $5. Earlier this month, county into three phases. a visible irregularity in the fl oor, He said the new plan would At 8 a.m. Saturday will commissioners asked architect He told commissioners that and a 2-inch gap has formed be- create a paved 18-space parking be the 5K Red Pepper Run, Warren Emo to estimate the total since the last meeting, structural tween the fl oor and baseboard in lot and an Americans with Dis- starting in front of the Blue renovation cost for the Coombs engineers found water damage in the southwest corner, he said. abilities Act-compliant entry on Parrot. Entry is $15 per Armory. their explorations of area under Emo said fl ooding around the the north side of the building. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Observer Training Manual National Marine Fisheries Service Southeast
Characterization of the US Gulf of Mexico and Southeastern Atlantic Otter Trawl and Bottom Reef Fish Fisheries Observer Training Manual National Marine Fisheries Service Southeast Fisheries Science Center Galveston Laboratory September 2010 TABLE OF CONTENTS National Overview ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Project Overview ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 8 Observer Program Guidelines and Safety ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 15 Observer Safety ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 15 Medical Fitness for Sea ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 15 Training ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 15 Before Deployment on Vessel ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 16 Seven Steps to Survival ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 18 Donning an Immersion Suit ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 20 Safety Aboard Vessels ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 22 Safety At‐Sea Transfers ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 23 Off‐Shore Communications ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 24 Advise to Women Going to Sea ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 27 Summary: What You Need to Know About Sea Survival ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 29 Deployment on Vessel -
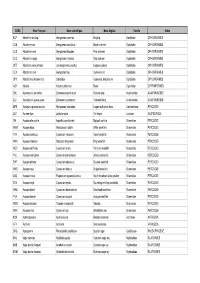
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

Invertebrate Food Selection
298 Volume 24: Mini Workshops Invertebrate Food Selection Catherine A. Teare Ketter1 and Jill M. Goldstein2 1School of Marine Programs 2Institute of Ecology Room 110J, Marine Sciences Building Ecology Building University of Georgia University of Georgia Athens, Georgia 30602-3636 Athens, Georgia 30602-2202 (706) 583-0862 (706) 542-2968 [email protected] [email protected] Catherine Teare Ketter received her B.S. and M.S. In Biology from the University of Alabama. She received her Ph.D. in research methodology and applied statistics in 1990 from the University of Alabama with an emphasis in the application of nonparametric multivariate techniques to biomedical and health behavioral data. Since 2000, Catherine has been responsible for developing the undergraduate introductory marine science laboratory courses at the University of Georgia. She holds a Master’s level teaching certificate in comprehensive science. Jill Goldstein received her B.A. in Biology from Binghamton University, her M.S. in Zoology from the University of South Florida, and is currently as doctoral candidate in Ecology at the University of Georgia. Her interests include animal behavior, field ornithology, and system ecology and modeling. Reprinted From: Teare Ketter, C. A. and J. M. Goldstein. 2003. Invertebrate food selection. Pages 298- 310, in Tested studies for laboratory teaching, Volume 24 (M. A. O’Donnell, Editor). Proceedings of the 24th Workshop/Conference of the Association for Biology Laboratory Education (ABLE), 334 pages. - Copyright policy: http://www.zoo.utoronto.ca/able/volumes/copyright.htm Although the laboratory exercises in ABLE proceedings volumes have been tested and due consideration has been given to safety, individuals performing these exercises must assume all responsibility for risk. -
20140421 SLIPSP Approvedplan.Pdf
St. Lucie Inlet Preserve State Park APPROVED Unit Management Plan STATE OF FLORIDA DEPARTMENT OF ENVIRONMENTAL PROTECTION Division of Recreation and Parks April 21, 2014 TABLE OF CONTENTS INTRODUCTION PURPOSE AND SIGNIFICANCE OF THE PARK ....................................... 1 Park Significance ............................................................................... 1 PURPOSE AND SCOPE OF THE PLAN .................................................... 2 MANAGEMENT PROGRAM OVERVIEW .................................................. 7 Management Authority and Responsibility ............................................. 7 Park Management Goals ..................................................................... 8 Management Coordination .................................................................. 9 Public Participation ............................................................................ 9 Other Designations ............................................................................ 9 RESOURCE MANAGEMENT COMPONENT INTRODUCTION ............................................................................... 11 RESOURCE DESCRIPTION AND ASSESSMENT .................................... 12 Natural Resources ........................................................................... 12 Topography ................................................................................. 12 Geology ...................................................................................... 15 Soils ......................................................................................... -

Observer Training Manual
Characterization of the US Gulf of Mexico and Southeastern Atlantic Otter Trawl and Bottom Reef Fish Fisheries Observer Training Manual National Marine Fisheries Service Southeast Fisheries Science Center Galveston Laboratory MARCH 2020 TABLE OF CONTENTS SECTION 1 ‐ INTRODUCTION National Overview ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐1 National Observer Program FAQ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐2 Penaeid Shrimp ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐3 Shrimp Observer Program Overview ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐4 Reef Fish Observer Program Overview ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐7 Observer Program Guidelines and Safety ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐10 Observer Safety ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐10 Medical Fitness for Sea ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐11 Training ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐11 Before Deployment on Vessel ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐11 Seven Steps to Survival ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐13 Donning an Immersion Suit ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐16 Safety aboard Vessels ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1‐18 General Safety Precautions ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ -

December 2005
NEWSFlorida Fossil Hunters Volume 15, Number 11 December 2005 Prez Sez...... Coming Events The auction items and our auctioneer Roy Singer are ready for our December 14th Christmas fossil buck auction and Christmas party. Wake up your 6:00pm Kid’s Fossil Blast dog and have him go dig up the old Mason jar that's buried in the 7:00pm Meeting and Auction back yard with your fossil bucks in it. January 3rd Please bring a dessert or your special covered dish to share at the Board Meeting party. The club will provide paper plates, cups, eating utensils, napkins, etc. January 18th Make a note on your calendar for 2006......our Fossil Fair will be on 7:00pm Meeting October 14th and 15th at the Central Florida Fairgrounds. The club needs 2 more board members to replace some current board members who have conflicting schedules. The officers up for re-election are: Dave Dunaway, President Paul Bordenkircher, Vice-President Sara Morey, Treasurer Board Members: Dave Dunaway, Ed Metrin, Roy Singer This season is a time for us to reflect on our place in this universe Table of Contents and be at peace with God. Fragments .....................................2 Dave Dunaway Kids FossilBlast .............................2 'Godzilla' Crocodile Had Head of a Dinosaur, Fins Like a Fish ..........3 Merry Christmas Old Bones Unearth New Date for Giant Deer’s Last Stand ...............4 Prehistoric Lizard Called Historic Link.....................................5 Don’t Miss this Month’s Meeting!! Membership Application ...................6 One week early – December 14th December 2005 Auction ................7 Calendar ........................................8 Florida Fossil Hunters News Florida Fossil Hunters News Volume 15, Number 11 - December 2005 Page 2 Fragments It'sTimetoRenew Be sure to renew your membership for 2006. -

Invertebrates
Invertebrates Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2014 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference.