Table 1A. Standard (Based on ISO and ASTM) Abbreviations of Plastics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Greenchem Industries Chemical and Solvent Product List A-Z
GreenChem Industries Chemical And Solvent Product List A-Z 1,4-Butanediol (BDO) Formic Acid, 80%, 85%, 90%, 95% N-Butanol 2-Ethylhexanol (2-EH) Gamma Butyrolactone (GBL) N-Butyl Acetate (BUTAC) 2-Ethylhexyl Acrylate (2-EHA) Glacial Acetic Acid (GAA) N-Heptane 911P Glutaraldehyde 50% N-Methyl Pyrrolidone (NMP) Acetic Acid Glycerin USP K & Tech Grade N-Propanol Acetone Glycol Ether DB N-Propyl Acetate Acetonitrile Glycol Ether DE Neopentyl Glycol (NPG) Adipic Acid Glycol Ether DM Nitromethane Alpha-Methylstyrene (AMS) Glycol Ether DPM Nonyl Phenol Asphalt Cutback Glycol Ether DPM Acetate (DPM Acetate) NP-9, NP-10, NP-12 Benzoic Acid Glycol Ether DPnB Odorless Mineral Spirits (OMS) Benzyl Alcohol USP & TECH Glycol Ether DPnP Oxalic Acid 99.6% Benzyl Chloride Glycol Ether EB P-Chlorbenzotrifluoride (PCBTF) Boric Acid Glycol Ether EEP Perchloroethylene (PERC) Butyl Acrylate Glycol Ether EM Phenol 85%, 90%, 99% Caustic Potash 90% Glycol Ether EP Phthalic Anhydride Glycol Ether EPH Polyethylene Glycol (PEG) Caustic Soda Flakes & Beads Glycol Ether PM Propylene Carbonate (PC) Citric Acid, USP Kosher Glycol Ether PMA Propylene Glycol USP (PG USP K) Cyclohexane Glycol Ether PnB Propylene Glycol Industrial (PGI) Cyclohexylamine Glycol Ether PnP Sebacic Acid Cylcohexanone Glycol Ether TPM Secondary Butanol (SBA) D-Limonene Glycolic Acid 70% Sodium Laurel Sulfate (SLES) 28%, 60%, 70% DB Acetate GreenCool (Inhibited Glycols) Solv 100 Diacetone Alcohol (DAA) Hexane Solv 150 Dibasic Ester (DBE) Hexylene Glycol (HG) Solv 200 Dibutyl Phthalate (DBP) Hydrochloric -

Vinyl Chloride
Withdrawn Provided for Historical Reference Only VINYL CHLORIDE Method no.: 04 Matrix: Air Target concentration: 1 ppm (2.5 mg/m3) (OSHA PEL) Procedure: Collection on charcoal (two-tubes in-series) , desorption with carbon disulfide, analysis by gas chromatography with a flame ionization detector. Stored in refrigerator and analyzed as soon as possible. Detection limit based on recommended air volume: 0.25 ppm Recommended air volume and sampling rate: 1 L at 0.05 L/min Standard error of estimate at the target concentration: 7.6% (Figure 4.1.) Status of method: Recommended by NIOSH, partially evaluated by OSHA Laboratory. Date:WITHDRAWN April 1979 Chemist: Dee R. Chambers Organic Methods Evaluation Branch OSHA Analytical Laboratory Salt Lake City Utah Note: OSHA no longer uses or supports this method (December 2019). Withdrawn Provided for Historical Reference Only 1. General Discussion 1.1. Background 1.1.1. History In January 1974, B.F. Goodrich Chemical Company informed NIOSH of several deaths among polyvinyl chloride production workers from angiosarcoma, a rare liver cancer. In response to this and other evidence of potential hazards, OSHA lowered the workplace air standard for vinyl chloride (VC) from 500 ppm to 1 ppm 8-h time weighted average. Since the recognition that VC was a carcinogen, a great deal of research into the sampling and analytical methodology has been conducted. Perhaps the most complete and thorough examination has been conducted by Hill, McCammon, Saalwaechter, Teass, and W oodfin for NIOSH titled "The Gas Chromatographic Determination of Vinyl Chloride in Air Samples Collected on Charcoal" (Ref. 5.1.). -

Chlorine Dioxide Compatibility Assessment
Chlorine Dioxide Compatibility Assessment DRF3366 - TECH-COMM-001 Created by: Tristel Solutions Limited, Lynx Business Park, Cambs, UK, CB8 7NY Copyright © Tristel Solutions T +44 (0) 1638 721500 – E [email protected] – W www.tristel.com January 2018 Key - Excellent - no change at all in the device - Good - slight cosmetic change/ decolourisation – material/device still fully functional - Fair – slight damage is observed– material/device still fully functional - Poor – visible pitting, breaking, brittle METALS Materials of construction 1 2 3 4 (Excellent) (Good) (Fair) (Poor) Stainless Steel 317 (Pure) X Stainless Steel 316 (pure, fully X austenitic with no ferric content) Stainless Steel 304 X (High grade, low ferric content) Hastelloy X Titanium X Aluminium X Aluminium (Anodised) X Brass X Copper X Chrome X Mild Steel X DRF3366 - TECH-COMM-001 Created by: Tristel Solutions Limited, Lynx Business Park, Cambs, UK, CB8 7NY Copyright © Tristel Solutions T +44 (0) 1638 721500 – E [email protected] – W www.tristel.com January 2018 PLASTICS Materials of construction 1 2 3 4 (Excellent) (Good) (Fair) (Poor) Perfluoroelastomer X PEEK X X Polypropylene X PVDF X X Polycarbonate X Polysulphone (natural) X Polysulphone (white) X PET-P X PMMA X Polyamide X Polyethylene X Polyoxymethylene X Acetal X ABS X Polymethylpentene (TPX) (PMP) X DRF3366 - TECH-COMM-001 Created by: Tristel Solutions Limited, Lynx Business Park, Cambs, UK, CB8 7NY Copyright © Tristel Solutions T +44 (0) 1638 721500 – E [email protected] – W www.tristel.com January 2018 PLASTICS -

2 Types of Noncrystalline Polymers
2 Types of Noncrystalline Polymers 1. Glassy polymer highly interpenetrated/entangled 2. Rubbery polymers random Gaussian coils Glass Transition Temperature εij Two viewpoints: • Increasing T: When kT > magnitude of εij, the thermal fluctuations can overcome local intermolecular bonds and the frozen (“glassy”) structure becomes “fluid-like”. • Decreasing T: As the temperature is lowered and T approaches Tg, the viscosity increases to ∞ and the material becomes “solid” Free Volume Theory of Tg Free volume, VF – extra space beyond what is present in an ordered crystalline packing (beyond the interstitial volume). VF(T) ≡ V(T) – V0(T) • V0 is occupied specific volume of atoms or molecules in the xline state and the spaces between them: ~ VXL. V(T) α • VF increases as T increases due to the Rate of cooling l difference in the thermal expansion V (T) αg XL coefficients (αg vs αl). FAST SLOW • V0(T) ≈ VXL(T) ↔ can take αg ≈αXL dV •V(T) = V (T ) + (T-T ) F T > T F F g g dT g Tg T • define fractional free volume, fF:Vf/V fF(T) = fF(Tg) + (T-Tg)αf αf = αl – αg Viewpoint: Tg occurs when available free volume drops below critical threshold for structural rearrangement [VITRIFICATION POINT], structure “jams up”. Tg Values of Amorphous Materials Table of representative amorphous solids, their bonding types, and their glass transition temperatures removed due to copyright restrictions. See Table 2.2 in Allen, S. M., and E.L. Thomas. The Structure of Materials. New York, NY: J. Wiley & Sons, 1999. Tg for Selected Polymers Glass Transition Temperature -

Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules
Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Cite as: Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 Published Online: 15 October 2009 Sharon G. Lias, Joel F. Liebman, and Rhoda D. Levin ARTICLES YOU MAY BE INTERESTED IN Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update Journal of Physical and Chemical Reference Data 27, 413 (1998); https:// doi.org/10.1063/1.556018 Density-functional thermochemistry. III. The role of exact exchange The Journal of Chemical Physics 98, 5648 (1993); https://doi.org/10.1063/1.464913 A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu The Journal of Chemical Physics 132, 154104 (2010); https://doi.org/10.1063/1.3382344 Journal of Physical and Chemical Reference Data 13, 695 (1984); https://doi.org/10.1063/1.555719 13, 695 © 1984 American Institute of Physics for the National Institute of Standards and Technology. Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules Sharon G. Lias Center for Chemical Physics, National Bureau of Standards, Gaithersburg, MD 20899 Joel F. Liebman Department of Chemistry, University of Maryland Baltimore County, Catonsville, MD 21228 and Rhoda D. Levin Center for Chemical Physics. National Bureau of Standards, Gaithersburg, MD 20899 The available data on gas phase basicities and proton affinities of molecules are compiled and evaluated. Tables giving the molecules ordered (1) according to proton affinity and (2) according to empirical formula, sorted alphabetically are provided. -

Blends of Polycarbonate Containing Fluorinated-Bisphenol-A and Polyvinyl Chloride
Europaisches Patentamt European Patent Office © Publication number: 0 576 057 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 93201533.2 int. Ci.5; C08L 69/00, C08L 27/06, C08G 64/10, //(C08L69/00, @ Date of filing: 28.05.93 27:06),(C08L27/06,69:00) © Priority: 01.06.92 US 891032 © Applicant: ENICHEM S.p.A. Piazza della Repubblica, 16 @ Date of publication of application: 1-20124 Milano(IT) 29.12.93 Bulletin 93/52 @ Inventor: Drzewinski, Michael A. © Designated Contracting States: 371 Clarksville Road, Princeton Junction AT BE CH DE DK ES FR GB GR IE IT LI LU MC New Jersey 08850(US) NL PT SE © Representative: Roggero, Sergio et al Ing. Barzano & Zanardo Milano S.p.A. Via Borgonuovo 10 1-20121 Milano (IT) © Blends of polycarbonate containing fluorinated-bisphenol-A and polyvinyl chloride. © Bisphenol A polycarbonate containing at least 15 mole % of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane (6F-Bisphenol A) can be blended with polyvinyl chloride (PVC) to form a thermodynamically miscible, transpar- ent, single phase blend at all compositions. Such blends are flame resistant as well as resistant to attack by acids, bases and many organic solvents. CO Rank Xerox (UK) Business Services (3. 10/3.6/3.3. 1) EP 0 576 057 A1 BACKGROUND OF THE INVENTION Field of the Invention: 5 This invention pertains to mixtures of polyvinyl chloride (PVC) and polycarbonates which contain at least 15 mole % of fluorinated bisphenol monomer units (F-PC) such as 2,2-bis-(4-hydroxyphenyl)- hexafluoropropane (6F-bisphenol A), herein referred to as 6F-PC. -

United States Patent (19) 11 Patent Number: 4,481,333 Fleischer Et Al
United States Patent (19) 11 Patent Number: 4,481,333 Fleischer et al. 45 Date of Patent: Nov. 6, 1984 54 THERMOPLASTIC COMPOSITIONS 58 Field of Search ................................ 525/192, 199 COMPRISING WINYL CHLORIDE POLYMER, CLPE AND FLUOROPOLYMER 56) References Cited U.S. PATENT DOCUMENTS 75) Inventors: Dietrich Fleischer, Darmstadt; Eckhard Weber, Liederbach; 3,005,795 10/1961 Busse et al. ......................... 525/199 3,294,871 2/1966 Schmitt et al. ...... 52.5/154 X Johannes Brandrup, Wiesbaden, all 3,299,182 1/1967 Jennings et al. ... ... 525/192 of Fed. Rep. of Germany 3,334,157 8/1967 Larsen ..................... ... 525/99 73 Assignee: Hoechst Aktiengesellschaft, Fed. 3,940,456 2/1976 Fey et al. ............................ 525/192 Rep. of Germany Primary Examiner-Carman J. Seccuro (21) Appl. No.: 566,207 Attorney, Agent, or Firm-Connolly & Hutz 22 Filed: Dec. 28, 1983 57 ABSTRACT 30 Foreign Application Priority Data The invention relates to a thermoplastic composition which comprises vinyl chloride polymers and chlori Dec. 31, 1982 (DE Fed. Rep. of Germany ....... 3248.731 nated polyethylene and which contains finely divided 51) Int. Cl. ...................... C08L 23/28; C08L 27/06; fluoropolymers and has a markedly improved process C08L 27/18 ability, particularly when shaped by extrusion. 52 U.S. C. .................................... 525/192; 525/199; 525/239 7 Claims, No Drawings 4,481,333 2 iaries and do not provide a solution to the present prob THERMOPLASTC COMPOSITIONS lem. COMPRISINGVINYL CHLORIDE POLYMER, -

SARAN™ Polyvinylidene Chloride (PVDC) Resins
Product Safety Assessment SARAN™ Polyvinylidene Chloride (PVDC) Resins Product Safety Assessment documents are available at www.dow.com/productsafety/assess/finder.htm. Select a Topic: Names Product Overview Manufacture of Product Product Description Product Uses Exposure Potential Health Information Environmental Information Physical Hazard Information Regulatory Information Additional Information References Names CAS Nos. 25038-72-6, 9011-06-7 Vinylidene chloride copolymer SARAN polyvinylidene chloride (PVDC) resins Vinylidene chloride/methyl acrylate copolymer SARAN resins Vinylidene chloride/vinyl chloride copolymer Back to top Product Overview SARAN™ resins are white, odorless granules.1 These resins are polyvinylidene chloride (PVDC) copolymers made from polymerizing vinylidene chloride with comonomers like vinyl chloride and alkyl acrylates.2 For further information, see Product Description. SARAN resins are used extensively in packaging applications for food, pharmaceuticals, hygiene products, and sterilized medical products. They offer excellent barrier performance to moisture, oxygen, and odors.3 For further information, see Product Uses. Because SARAN resins are used extensively in food packaging, it is possible for consumers to come into contact with them. Workplace exposure is also possible.4 For further information, see Exposure Potential. SARAN resins are essentially nonirritating to the eyes and skin. Dust from SARAN products may cause temporary mechanical irritation to the skin and eyes under extreme conditions. However, the products are considered to present no significant health hazard.5 For further information, see Health Information. SARAN resins are expected to be inert in the environment. They are unlikely to accumulate in the food chain, and are practically nontoxic to aquatic organisms on an acute basis.6 For further information, see Environmental Information. -
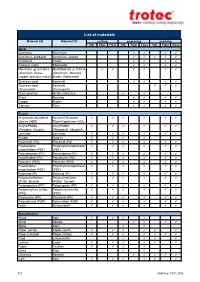
List of Materials
List of materials Material EN Material DE cutting engraving marking CO2 Fiber Flexx CO2 Fiber Flexx CO2 Fiber Flexx Metal Aluminum Aluminium Aluminum, anodized Aluminium, eloxiert Chromium Chrom Precious metal Edelmetall Metal foils up to 0.5mm Metallfolie bis zu 0,5mm (Aluminum, Brass, (Aluminium, Messing, Copper, precious metal) Kupfer, Edelmetall) Stainless steel Edelstahl Stainless steel Edelstahl (Thermark®) (Thermark®) Metal, painted Metall, lackiertes Brass Messing Copper Kupfer Titanium Titan Plastic Acrylonitrile butadiene Acrylnitril-Butadien- styrene (ABS) Styrol-Copolymer (ABS) Acrylic/PMMA Acryl/PMMA (Plexiglas, Altuglas, (Plexiglas®, Altuglas®, LaminatePerspex) LaminatePerspex®) Rubber Gummi Polyamide (PA) Polyamid (PA) Polybutylene Polybutylenterephthalat terephthalate (PBT) (PBT) Polycarbonate (PC) Polycarbonat (PC) Polyethylene (PE) Polyethylen (PE) Polyester (PES) Polyester (PES) Polyethylene Polyethylenterephthalat terephthalate (PET) (PET) Polyimide (PI) Polyimid (PI) Polyoxymethylene Polyoxymethylen (POM) -Delrin® (POM) - Delrin® Polypropylene (PP) Polypropylen (PP) Polyphenylene sulfide Polyphenylensulfid (PPS) (PPS) Polystyrene (PS) Polystyrol (PS) Polyurethane (PUR) Polyurethan (PUR) Foam Schaumstoff Miscellanious Wood Holz Mirror Spiegel Stone Stein Paper (white) Papier (weiß) Paper (colored) Papier (färbig) Food Lebensmittel Leather Leder -

Plastic Materials List Original.Xlsx
Partec Institute 1030 Cavendish Rd. Mt. Gravatt East. Brisbane Qld 4122 Ph 07 3849 7878. www.partec.qld.edu.au ABREVIATIONS OF POLYMER MATERIALS AAS acrylonitrile acrylic styrene PE polyethylene ABS acrylonitrile butadiene styrene PEO poly (ethylene oxide) AES acrylonitrile ethylene ester PEP polyethylene propylene A/MMA acrylonitrile / methyl methacrylate PESU polyether sulphone AS acrylonitrile styrene PET polyethylene terephthalate ASA acrylonitrile styrene acrylate PETP crystaline pet (tetramethylene terephthalate) BMC bulk moulding compound PEX polyethylene crosslinked CA cellulose acetate PF phenol formaldehyde resin CAB cellulose acetate butyrate PI polyimide CAP cellulose acetate propionate PIB polyisobutylene CF cresol formadehyde PMMA polymethyl methacrylate CLPE chlorinated polyethylene PMP poly (4-methylpentene- I ) CLPVC /CPVC chlorinated polyvinyl chloride POB polypoxybenzoate CMC carboxymethyl cellulose POM polyoxymethylene (polyacetal) CN cellulose nitrate PP polypropylene CP cellulose propionate PPC polypropylene compound CPE chlorinated polyethylene PPE polypropylene ethylene CS casein PPF polypropylene foam DAP diallyl phthalate PPO polyphenylene oxide modified DMC dough molding compound PPDX polypropylene oxide EC ethyl cellulose PPS polyphenylene sulphide E/EA, EEA ethylene / ethyl acetate PPSU polyphenylene sulphone EEBC polyester (ether ester block copolymer) PS polystyrene E / MA ethylene methacrylate acid PSU polysulphone EP epoxide, epoxy resin PTFE polytetrafluoethylene EPS polystyrene - expandable ethylene -

Table 1. SOME NAMES and ABBREVIATIONS of Plastics and Elastomers
TABlE 1. SOME NAMES AND ABBREVIATIONS OF PlASTICS AND ElASTOMERS. Common name Abbreviation Acetal (homopolymer and copolymer) POM-H and POM-K Acrylate styrene acrylonitrile ASAorAAS Acrylate modified styrene acrylonitrile ASAorAAS Acrylic acid ester rubber ACM Acrylonitrile butadiene rubber or nitrile butadiene rubber NBR Acrylonitrile butadiene styrene ABS Acrylonitrile styrene/chlorinated polyethylene ACS Acrylonitrile methyl methacrylate AMMA Acrylonitrile styrene/EPR rubber or, acrylonitrile ethylene propylene styrene AES Alpha methyl styrene AMS Atactic polypropylene APPorPP-A Butadiene rubber or, cis-1,4-polybutadiene rubber or, polybutadiene rubber BR Butadiene styrene block copolymer BDS Butyl rubber IIR Bulk molding compound BMC Casein formaldehyde CF Cellulose acetate CA Cellulose acetate butyrate CAB Cellulose acetate propionate CAP Cellulose nitrate CN Chlorinated polyethylene CPEorCM Chlorinated polyvinyl chloride CPVC or, PVC-C Chloro-polyethylene or, chlorinated polyethylene. CM or CPE or, PE-C Chloroprene rubber or, polychloroprene rubber CR Chlorotrifluoroethylene ethylene copolymers ECfFE Cis-polyisoprene or, cis-1,4-polyisoprene IR Coumarone indene resins CIR Diallyl phthalate DAP Diallyl isophthalate DAIP Dough molding compound DMC Elastomeric alloy melt processable rubber EA-MPR Elastomeric alloy thermoplastic vulcanizate EA-TPV Epichlohydrin rubber CHR Epoxy or, epoxide EP Epoxy or, epoxide, with glass fiber EPGF Ethyl cellulose EC Ethylene acryic acid EAA Ethylene propylene diene monomer (an EPR terpolymer) EPDM -

Synthetic Polymeric Materials for Bone Replacement
Review Synthetic Polymeric Materials for Bone Replacement Mônica Rufino Senra and Maria de Fátima Vieira Marques * Technology Center, Bloco J, Instituto de Macromoleculas Professora Eloisa Mano, Universidade Federal do Rio de Janeiro, IMA-UFRJ, Av. Horacio Macedo, 2030, Rio de Janeiro RJ 21941-598, Brazil; [email protected] * Correspondence: [email protected] Received: 17 November 2020; Accepted: 18 December 2020; Published: 19 December 2020 Abstract: Some treatment options available to repair bone defects are the use of autogenous and allogeneic bone grafts. The drawback of the first one is the donor site’s limitation and the need for a second operation on the same patient. In the allograft method, the problems are associated with transmitted diseases and high susceptibility to rejection. As an alternative to biological grafts, polymers can be used in bone repair. Some polymers used in the orthopedic field are poly(methyl methacrylate), poly(ether-ether-ketone), and ultra-high molecular weight polyethylene (UHMWPE). UHMWPE has drawn much attention since it combines low friction coefficient and high wear and impact resistance. However, UHMWPE is a bioinert material, which means that it does not interact with the bone tissue. UHMWPE composites and nanocomposites with hydroxyapatite (HA) are widely studied in the literature to mitigate these issues. HA is the main component of the inorganic phase in the natural bone, and the addition of this bioactive filler to the polymeric matrix aims to mimic bone composition. This brief review discusses some polymers used in orthopedic applications, focusing on the UHMWPE/HA composites as a potential bone substitute. Keywords: poly(methyl methacrylate); poly(ether-ether-ketone); ultra-high molecular weight polyethylene; hydroxyapatite; orthopedic applications 1.