Leprosy ROBERT C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Volume 61, Number 4, December 1990 Published Quarterly for The
Volume 61, Number 4, December 1990 LEPROSY REVIEW Published Quarterly for the British Leprosy Relief Association ISSN 0305-7518 Leprosy Review A journal contributing to the better understanding of leprosy and its control British Leprosy Relief Association LEPRA Editorial Board PRO~ESSOR J . L. TURK (Chairman and Edilor) DR R . J . W . R EES , C.M .G . (Vice-Chairman) The Royal Coll ege of Surgeons National Institute fo r Medical Research Department of Pa thology, The Ridgeway 35- 43 Lincoln's Inn Field Mill Hill, London NW7 IAA London W C2A 3PN JANE EVILLE, M .B.E. DR M . J . COLSTON 5 Sandall Close National Institute for Medical Research Ealin g The Ridgeway, Mill Hill Lo ndo n W 5 IJ E London NW7 I AA PROFESSOR P. E. M . FINE DR PATRI CIA ROSE Department of Epidemiology Allendale Ho use and Populati on Sciences A ll endale Road London School of H ygiene Hexha m N E46 2DE and Tropical Medicine Keppel Street DR M . F. R . WATERS , O .B. E. London W C I E 7HT Hospital fo r Tro pical Diseases DR S. LUCAS 4 St Pa ncras W ay School of Medicine Lo ndo n NW l OPE University Col1ege and Middlesex Medical School, London DR H . W . WH EATE , O .B.E. U ni versi ty Street 50 Avenue Road, Belmo nt, Sulto n Lo ndo n W C IE 6JJ Surrey SM2 6JB Editorial Office: Lepra, Fa irfax House, Causton Road, Colchester C01 1 PU , England Assistant Editor: Jennet Batten, 94 Church Road, Wheatley, Oxon OX9 1 LZ, England Leprosy Review is published by the British Leprosy Relief Association (LEPRA) with the main objective of contributing towards the better understanding of leprosy a nd its control. -
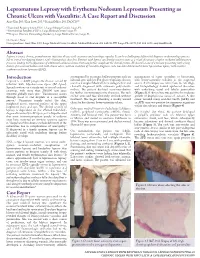
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Leprosy' Revi"Ew
LEPROSY' REVI"EW lbe QD8rterIy Publication of THE BRITISH LEPROSY RELIEF ASSOCIAll0N VOL. XXVIII. No 3. JULY 1957 Principal Contents EditoriaIs Secondary Infections and Neoplasms in Leprosy Patients. Leprosy of the Eye. Thiosemicarbazone in the Treatment of the Reactional and Bordeline Forms of Leprosy. Some Data on the Influence of B.C.G. Vaccination in Leprosy Patients. Abstracts 8 PORTMAN STREET, LONDON, W.l Price: Three Shillings and Sixpence, plus posfage Annual Subscripfion: Fiffeen Shillings, including posfage LEPROSY REVIEW VOL. XXVIII, No. 3. JULY, 1957 CONTENTS PAGE Editorials: Corticosteroids in Leprosy 91 Is there a place for hypnotherapy in leprosy treatment? 92 Secondary Infections and Neoplasms in Leprosy Patients FELIX CONTRERAS 95 Leprosy of the Eye .. W. ]. HOLMES 108 Thiosemicarbazone in the Treatment of the Reactional and Borderline Forms of Leprosy H. W. WHEATE 124 Some Data on the Influence of BCG Vaccination in Leprosy Patients ]. VAN DE HEYNING 130 Abstracts 131 Edited by DR. J. Ross INNES, Medical Secretary of the British Leprosy Relief Association, 8 Portman Street, London, W.l, to whom all communications should be sent. The Association does not accept responsibilty for views expressed by writers. Contributors of original articles will receive 25 loose reprints free, but more formal bound reprints must be ordered at time of submitting the article, and the cost reimbursed later. THE ONE DOSE REMEDY , ANTEPAR ' is such a simple and practical ascarifuge, it enables you to tackle tbe roundworm problem on a large scale. You simply givc each adult or child onc dosc of 'A TEPAR' }!'Iixir. 0 purging, fasting or dieling is necessary. -

Introduction to Bacteriology and Bacterial Structure/Function
INTRODUCTION TO BACTERIOLOGY AND BACTERIAL STRUCTURE/FUNCTION LEARNING OBJECTIVES To describe historical landmarks of medical microbiology To describe Koch’s Postulates To describe the characteristic structures and chemical nature of cellular constituents that distinguish eukaryotic and prokaryotic cells To describe chemical, structural, and functional components of the bacterial cytoplasmic and outer membranes, cell wall and surface appendages To name the general structures, and polymers that make up bacterial cell walls To explain the differences between gram negative and gram positive cells To describe the chemical composition, function and serological classification as H antigen of bacterial flagella and how they differ from flagella of eucaryotic cells To describe the chemical composition and function of pili To explain the unique chemical composition of bacterial spores To list medically relevant bacteria that form spores To explain the function of spores in terms of chemical and heat resistance To describe characteristics of different types of membrane transport To describe the exact cellular location and serological classification as O antigen of Lipopolysaccharide (LPS) To explain how the structure of LPS confers antigenic specificity and toxicity To describe the exact cellular location of Lipid A To explain the term endotoxin in terms of its chemical composition and location in bacterial cells INTRODUCTION TO BACTERIOLOGY 1. Two main threads in the history of bacteriology: 1) the natural history of bacteria and 2) the contagious nature of infectious diseases, were united in the latter half of the 19th century. During that period many of the bacteria that cause human disease were identified and characterized. 2. Individual bacteria were first observed microscopically by Antony van Leeuwenhoek at the end of the 17th century. -

Histopathological Study of Dermal Granuloma Gunvanti B
https://ijmsweb.com Indian Journal of Medical Sciences Original Article Histopathological study of dermal granuloma Gunvanti B. Rathod1, Pragnesh Parmar2 1Departments of Pathology, 2Forensic Medicine, GMERS Medical College, Vadnagar, Gujarat, India. ABSTRACT Introduction: The objectives of this study were to confirm the diagnosis of clinically suspected dermal granuloma- tous diseases by histopathological examination and by routine and special stains as well as to study the incidence of various types of dermal granulomas. *Corresponding author: Materials And Methods: This study was conducted at the Department of Pathology in collaboration with De- partment of Skin and Venereal disease. A total of 90 cases from outdoor patient department of skin and venereal Dr. Gunvanti Rathod, disease, which were clinically diagnosed as suspected dermal granulomatous diseases, were taken as the study Departments of Pathology, population. GMERS Medical College, Vadnagar, Gujarat, India. Results: In our study, we found that leprosy had the highest incidence (50%), followed by cutaneous tuberculosis (30%) among all dermal granulomatous diseases like syphilis, fungal, granuloma annulare, foreign body, actino- [email protected] mycosis, and sarcoidosis. Dermal granulomas were most common in middle age between 21 and 40 years of age. Received : 18 September 19 Conclusion: Histopathology played an important role in the final diagnosis of dermal granulomatous lesions. Most common dermal granulomatous disease was leprosy, followed by cutaneous tuberculosis. -

Medical Bacteriology
LECTURE NOTES Degree and Diploma Programs For Environmental Health Students Medical Bacteriology Abilo Tadesse, Meseret Alem University of Gondar In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education September 2006 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2006 by Abilo Tadesse, Meseret Alem All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. PREFACE Text book on Medical Bacteriology for Medical Laboratory Technology students are not available as need, so this lecture note will alleviate the acute shortage of text books and reference materials on medical bacteriology. -

Commuin ICABLE DI SEASES STUDENT TEXT 1980
COMMUiN I CABLE DI SEASES STUDENT TEXT 1980 Rural Health Development Project Ministry of Health and Social Welfare Maseru, Lesotho ACK NOWLE;DGEMEN:'TS Nurse C.inician tVaini.nq mateL ial :;are Lesotho adaptations based upon the ME:DiEX proLotype curriculum for L'a.inin mid-Lo vol health workers. ['le prototype MiDEX matLerials 'or developed by Lhe Halth Manpowe r DovelO\opient Sta :ff of the ,Iohn A.Itirls School Med f iie, Univrsity of Iawai . The or.'ig.nili .1 prototypeS we re based on ttraini.nq ex2.U, IiiOn Ce in over a dozen third-world ccuntrios. These were reviaed on the basis of MDS experienaace in Micronesia, Till.and, Pakistan, and Guy ana beftore being made availab.Le to Lesotho under ai UI.S.A.I.D. funded 'ontract. Major adaptation in lesotho began at: the National Nurse Clini~cian T'ira. ninq " ,oqraimmo Curr iculum Adaptation Works:l'.hopt ld a, , 'Mzv.od in ,.nuary L98G. The ncar.y Li fty parti2i.paniLa uce senLtd alI majcr halth and ;i'ualth related ativ iuLits in Lesotho, hoth G ove rnienL and prIvate. h'iie'e participants and othrs workinj as irdividuas and tLhen as rvrev, i commi tees have adapted the Nurse Cli.niciai traini 1 aterLj.sL to eeLt the conditions and nee:ds of Lesoatho. The 6overnment of lenotho and particularly the staff of the Nurse C linir'i.an traini.ing 'rogrmme are grateful to IlMDS for :supilyin, the proottype materials and to a].]. thos individuals h.;Io have nelped in the Lesotho adaptation ioI. -

Histopathological Study of Granulomatous Dermatoses - a 2 Year Study at a Tertiary Hospital
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Histopathological Study of Granulomatous Dermatoses - A 2 Year Study at a Tertiary Hospital Velpula Nagesh Kumar1*, Kotta. Devender Reddy2**, N Ezhil Arasi3** 1Tutor, 2Associate Professor, 3Professor & Head, *Department of Pathology, Rajiv Gandhi Institute of Medical Sciences (RIMS), Govt. Medical Collage, Kadapa, Andhra Pradesh. **Department of Pathology, Osmania Medical Collage, Hyderabad, Telangana. Corresponding Author: Velpula Nagesh Kumar Received: 14/07/2016 Revised: 10/08/2016 Accepted: 11/08/2016 ABSTRACT Granulomatous inflammation is a type of chronic inflammation that has distinctive pattern of presentation with wide etiology and can involve any organ. Pathologists come across this lesion frequently and through knowledge of granulomatous lesions are very much essential to discriminate them from other lesions in the skin as they closely mimic each other. The aim of the present study is know the types of dermal granulomas, their prevalence, age and sex distribution, modes of presentation and histopathological spectrum. This prospective study was undertaken at Osmania General Hospital, Hyderabad from June 2012 to May 2014. A total of 620 skin biopsies were received at the Department of Pathology, histopathological sections of all the cases were critically analyzed and were classified on a “pattern based” approach according to Rabinowitz and Zaim et al. 172 cases were categorized histopathologically as granulomatous dermatoses. Granulomatous dermatoses were more common in males and the peak age of incidence was in 3rd decade. Incidence of Granulomatous dermatoses was 27.7% which was comparable with available literature. In the present study we found that Infections form an important cause of granulomatous dermatoses with majority of cases being leprosy followed by cutaneous tuberculosis and foreign body granulomas. -

Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe Between 2009 and 2018
microorganisms Article Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe between 2009 and 2018 Anna Beltrame 1,* , Gianfranco Barabino 2, Yiran Wei 2, Andrea Clapasson 2, Pierantonio Orza 1, Francesca Perandin 1 , Chiara Piubelli 1 , Geraldo Badona Monteiro 1, Silvia Stefania Longoni 1, Paola Rodari 1 , Silvia Duranti 1, Ronaldo Silva 1 , Veronica Andrea Fittipaldo 3 and Zeno Bisoffi 1,4 1 Department of Infectious, Tropical Diseases and Microbiology, I.R.C.C.S. Sacro Cuore Don Calabria Hospital, Via Sempreboni 5, 37024 Negrar di Valpolicella, Italy; [email protected] (P.O.); [email protected] (F.P.); [email protected] (C.P.); [email protected] (G.B.M.); [email protected] (S.S.L.); [email protected] (P.R.); [email protected] (S.D.); [email protected] (R.S.); zeno.bisoffi@sacrocuore.it (Z.B.) 2 Dermatological Clinic, National Reference Center for Hansen’s Disease, Ospedale Policlinico San Martino, Sistema Sanitario Regione Liguria, Istituto di Ricovero e Cura a Carattere Scientifico per l’Oncologia, Largo Rosanna Benzi 10, 16132 Genoa, Italy; [email protected] (G.B.); [email protected] (Y.W.); [email protected] (A.C.) 3 Oncology Department, Mario Negri Institute for Pharmacological Research I.R.C.C.S., Via Giuseppe La Masa 19, 20156 Milano, Italy; vafi[email protected] 4 Department of Diagnostic and Public Health, University of Verona, P.le L. A. Scuro 10, 37134 Verona, Italy * Correspondence: [email protected]; Tel.: +39-045-601-4748 Received: 30 June 2020; Accepted: 23 July 2020; Published: 24 July 2020 Abstract: Leprosy is a chronic neglected infectious disease that affects over 200,000 people each year and causes disabilities in more than four million people in Asia, Africa, and Latin America. -

5 Allergic Diseases (And Differential Diagnoses)
Chapter 5 5 Allergic Diseases (and Differential Diagnoses) 5.1 Diseases with Possible IgE Involve- tions (combination of type I and type IVb reac- ment (“Immediate-Type Allergies”) tions). Atopic eczema will be discussed in a separate section (see Sect. 5.5.3). There are many allergic diseases manifesting in The maximal manifestation of IgE-mediated different organs and on the basis of different immediate-type allergic reaction is anaphylax- pathomechanisms (see Sect. 1.3). The most is. In the development of clinical symptoms, common allergies develop via IgE antibodies different organs may be involved and symp- and manifest within minutes to hours after al- toms of well-known allergic diseases of skin lergen contact (“immediate-type reactions”). and mucous membranes [also called “shock Not infrequently, there are biphasic (dual) re- fragments” (Karl Hansen)] may occur accord- action patterns when after a strong immediate ing to the severity (see Sect. 5.1.4). reactioninthecourseof6–12harenewedhy- persensitivity reaction (late-phase reaction, LPR) occurs which is triggered by IgE, but am- 5.1.1 Allergic Rhinitis plified by recruitment of additional cells and 5.1.1.1 Introduction mediators.TheseLPRshavetobedistin- guished from classic delayed-type hypersensi- Apart from being an aesthetic organ, the nose tivity (DTH) reactions (type IV reactions) (see has several very interesting functions (Ta- Sect. 5.5). ble 5.1). It is true that people can live without What may be confusing for the inexperi- breathing through the nose, but disturbance of enced physician is familiar to the allergist: The this function can lead to disease. Here we are same symptoms of immediate-type reactions interested mostly in defense functions against are observed without immune phenomena particles and irritants (physical or chemical) (skin tests or IgE antibodies) being detectable. -

LEARNING from LEPROSY Be Enjoyed by 50%Of the Urbanpopulation, but Only 15% Monoclonal Anti-Interferon (IFN)-Y Antibodies
0022- 1767/86/137 1 -0OOiSO2.00/0 THEJOURNAL OF 1MMUNOLoGY Vol. 137. No. 1. July 1, I986 Copyright 0 1986 by The American Association of Immunol~lsts Prlnted In U.S.A. American Associationof Immunologists PRESIDENTIALADDRESS LEARNINGFROM LEPROSY:A PERSPECTIVE ONIMMUNOLOGY AND THE THIRDWORLD BARRY R. BLOOM From the Departmentsof Microbiology and Immunology. andCell Biology, Albert Einstein Collegeof Medicine. Bronx,NY 10461 "If we take the widest and wisest view of a Cause. there is no such thingas a Lost Cause, because there is no such thingas a Gained Cause. We fight for Lost Causes because we know that our defeat and dismay may be the preface to our successors' victory, although that victory itself will be temporary; we fi ht rather to keep somethning alive than in the expectation t fl at anything will triumph. "T.S. Eliot "A Map of the World Without Utopia on It Is not Worth Glancing At." "Oscar Wilde Let mebegin with a case history. notof an individual. tussis,tetanus, tuberculosis, polio, and measles, and but ratherof a country. any of the fortypoorest nations consequently 0.5%of them became lame from polio, 1% on earth. Let me ask you to try to imagine our qualityof died from neonatal tetanus. 2% succumbedto whooping life, if life expectancy at birth in this countrywere 42 yr. cough. and 3%died from measles. We would be living in if infant mortality at birth were 140 per thousand.if 40% a country whose average gross national product per cap- of our children suffered from malnutrition. and if only ita would be $310/yr: in which 37% of males, but only 10%of children were immunized against diphtheria, per-14% of females, would be literate.