Handheld Raman Library
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Provisional Peer-Reviewed Toxicity Values for Sulfolane (Casrn 126-33-0)
EPA/690/R-12/024F l Final 1-30-2012 Provisional Peer-Reviewed Toxicity Values for Sulfolane (CASRN 126-33-0) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Dan D. Petersen, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY ICF International 9300 Lee Highway Fairfax, VA 22031 PRIMARY INTERNAL REVIEWERS Ghazi Dannan, PhD National Center for Environmental Assessment, Washington, DC Q. Jay Zhao, PhD, MPH, DABT National Center for Environmental Assessment, Cincinnati, OH This document was externally peer reviewed under contract to Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). i Sulfolane TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS ................................................................................... iii BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING PPRTVS ...........................................................................................1 -

Identification of Promising Alternative Mono-Alcohol Fuel Blend
energies Article Identification of Promising Alternative Mono-Alcohol Fuel Blend Components for Spark Ignition Engines Saeid Aghahossein Shirazi 1, Thomas D. Foust 1,2 and Kenneth F. Reardon 1,2,* 1 Department of Chemical and Biological Engineering, Colorado State University, Fort Collins, CO 80523, USA; [email protected] (S.A.S.); [email protected] (T.D.F.) 2 National Renewable Energy Laboratory, Golden, CO 80401, USA * Correspondence: [email protected] Received: 24 March 2020; Accepted: 12 April 2020; Published: 15 April 2020 Abstract: Alcohols are attractive fuel blendstocks for spark ignition engines due to their high octane values and potentially positive influence on performance and emission. Although methanol, ethanol, and butanol have been widely studied, other biomass-derived alcohols may have similar or better properties. However, it is not feasible to experimentally investigate the fuel potential of every molecule. The goals of this study were to develop a methodology for rapid screening of a fuel property database for mono-alcohols and to identify alcohols with the potential of blending to produce advantaged motor gasolines. A database was developed with 13 fuel properties of all saturated C1–C10 mono-alcohols. A decision framework was used to evaluate alcohols suitable for blending in gasoline for spark ignition engines in two scenarios: low-range (up to 15 vol%) blends and high-range (greater than 40 vol%) blends. The low-range blend cases resulted in the identification of 48 alcohols. In the case of high-range blending, only six alcohols were found to be suitable. This is the first study to systematically evaluate all C1–C10 saturated alcohols for blending with gasoline using relevant fuel properties. -

Toxicological Basis Data for the Derivation of EU-LCI Values For
TEXTE 223/2020 Toxicological basis data for the derivation of EU- LCI values for neopentyl glycol, diisobutyl succinate, diisobutyl glutarate, 1,2- dimethoxyethane and 1,2-diethoxyethane Final report German Environment Agency TEXTE 223/2020 Ressortforschungsplan of the Federal Ministry for the Enviroment, Nature Conservation and Nuclear Safety Project No. (FKZ) 3719 62 205 0 Report No. FB000359/ENG Toxicological basis data for the derivation of EU-LCI values for neopentyl glycol, diisobutyl succinate, diisobutyl glutarate, 1,2- dimethoxyethane and 1,2-diethoxyethane Final report by Dr. Barbara Werschkun Wissenschaftsbüro, Berlin On behalf of the German Environment Agency Imprint Publisher Umweltbundesamt Wörlitzer Platz 1 06844 Dessau-Roßlau Tel: +49 340-2103-0 Fax: +49 340-2103-2285 [email protected] Internet: www.umweltbundesamt.de /umweltbundesamt.de /umweltbundesamt Report performed by: Wissenschaftsbüro Dr. Barbara Werschkun Monumentenstr. 31a 10829 Berlin Germany Report completed in: May 2020 Edited by: Section II 1.3 Indoor Hygiene, Health-related Environmental Impacts Dr. Ana Maria Scutaru Publication as pdf: http://www.umweltbundesamt.de/publikationen ISSN 1862-4804 Dessau-Roßlau, December 2020 The responsibility for the content of this publication lies with the author(s). TEXTE Toxicological basis data for the derivation of EU-LCI values for neopentyl glycol, diisobutyl succinate, diisobutyl glutarate, 1,2-dimethoxyethane and 1,2-diethoxyethane – Final report Abstract: Toxicological basis data for the derivation of EU-LCI values for neopentyl glycol, diisobutyl succinate, diisobutyl glutarate, 1,2-dimethoxyethane and 1,2-diethoxyethane The objective of this study was the evaluation of toxicological data for five substances as basis for the derivation of EU-LCI values. -

Mechanistic Study of Methylbenzene Dealkylation in Methanol-To-Olefins
Journal of Catalysis 369 (2019) 86–94 Contents lists available at ScienceDirect Journal of Catalysis journal homepage: www.elsevier.com/locate/jcat Mechanistic study of methylbenzene dealkylation in methanol-to-olefins catalysis on HSAPO-34 ⇑ Andrew Hwang, Blake A. Johnson, Aditya Bhan Department of Chemical Engineering and Materials Science, University of Minnesota, Minneapolis, MN 55455, USA article info abstract Article history: Methylbenzenes entrained within the cavities of silicoaluminophosphate zeotype HSAPO-34 react with Received 14 June 2018 methanol in H+-mediated dealkylation to give ethylene and propylene in methanol-to-olefins catalysis. Revised 1 August 2018 Methylbenzenes dealkylation on solid acids is proposed to occur either via the side-chain mechanism, Accepted 14 October 2018 where an exocyclic C@C undergoes successive methylation prior to CAC cleavage for olefin elimination, or the paring mechanism, where ring contraction to a bicyclohexenyl cation precedes CAC cleavage for olefin elimination. Distinct dealkylation mechanisms prescribe distinct combinations of C atoms—from Keywords: aromatic methyl, aromatic ring, and methanol/dimethyl ether—to comprise the olefin product. Site- Methanol-to-olefins specific isotope tracing that distinguishes between isotope labels in aromatic methyl and aromatic ring Methylbenzenes dealkylation Isotope tracing positions for each methylbenzene shows that tetramethylbenzene gives ethylene via the side-chain Site-specific mechanism and penta- and hexamethylbenzene give propylene via the paring mechanism. The ratio of Quantitative 13C NMR propylene selectivity to ethylene selectivity increases in methanol reactions on HSAPO-34 entrained with HSAPO-34 a distribution of methylbenzenes deliberately manipulated towards increasing fractions of penta- and Methylbenzene flash chromatography hexamethylbenzene, corroborating the conclusion that aromatic precursors and dealkylation mecha- nisms for ethylene diverge from those for propylene. -

Groundwater and Soil Oxygen and Nutrients (Soil Tilling, Blowers) (Biogenie, 2006)
RemTech 2016 Brent Lennox, M.Sc., P.Geol., Senior Hydrogeologist Eric Pringle, M.A.Sc., P.Eng., Principal Hydrogeological Engineer Waterline Resources Inc. Used in Sulfinol for sour gas sweetening since 1960s Human health related guidelines Poorly adsorbed to soil High solubility in water Microbial degradation slow in typical groundwater conditions Clear, colourless, no field indicators (visual or olefactory) Microbial degradation rapid in aerobic environments and surface water (CCME, 2006) + 2- C4H8O2S + 6.5O2 4CO2 + 3H2O + 2H +SO4 Nutrients improve degradation times Low pH conditions inhibit degradation Typical degradation times: 2 to 4 days at 28°C and 8 to 12 days at 8°C (Green et al., 1998), average air temperatures during trials ranged from 6.9 to 14.1°C Groundwater and Soil Oxygen and nutrients (soil tilling, blowers) (Biogenie, 2006) Groundwater Activated Sludge Treatment System (WorleyParsons Komex, 2008) Oxidants (e.g., hydrogen peroxide) and/or UV light Mixed success (Barr Engineering, 2013; Gallegos et al., 2013; EBA, 2015) Peroxide and iron catalyst shown to be more effective than peroxide (Gallegos, 2013) No sulphate as by-product Site is an operating gas plant located in southern Alberta Constructed in 1960s Sulfolane investigation and monitoring since 1994 No active facilities Downgradient of active facilities Majority of plant waste stored here before the 1980s Potential materials disposed: alumina catalyst, filters (compressor, sulfinol, salt water, glycol, solvent receiver), zeolite, etc. Cells -

Final Scope of the Risk Evaluation for Formaldehyde CASRN 50-00-0
EPA Document# EPA-740-R-20-014 August 2020 United States Office of Chemical Safety and Environmental Protection Agency Pollution Prevention Final Scope of the Risk Evaluation for Formaldehyde CASRN 50-00-0 August 2020 TABLE OF CONTENTS ACKNOWLEDGEMENTS ......................................................................................................................6 ABBREVIATIONS AND ACRONYMS ..................................................................................................7 EXECUTIVE SUMMARY .....................................................................................................................11 1 INTRODUCTION ............................................................................................................................14 2 SCOPE OF THE EVALUATION ...................................................................................................14 2.1 Reasonably Available Information ..............................................................................................14 Search of Gray Literature ...................................................................................................... 15 Search of Literature from Publicly Available Databases (Peer-reviewed Literature) ........... 16 Search of TSCA Submissions ................................................................................................ 24 2.2 Conditions of Use ........................................................................................................................25 Conditions of Use -

Catalog Feb 2007 Final.Pmd
Table of Contents How To Use This Catalog ............................................................................................... 2 Service Information .................................................................................................... 3-4 Placing Orders - Shipping........................................................................................................3 Miscellaneous Information......................................................................................................4 Product Information................................................................................................. 5-17 Definitions .............................................................................................................. 5 Detergent Analysis ............................................................................................. 6-7 Lipid-like Detergents.......................................................................................... 8-9 FOS-CHOLINE® Detergents .............................................................................................8 FOS-MEA® ...........................................................................................................................9 Miscellaneous ....................................................................................................................9 Nonionic Detergents ......................................................................................10-14 Sugar-based Detergents ....................................................................................... -

Diglycidyl Ethers
Europaisches Patentamt (19) J European Patent Office Office europeen des brevets (11) EP0 911 326 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC (43) Date of publication: (51) Int. CI.6: C07D 303/27, C08G 59/04 28.04.1999 Bulletin 1999/17 (86) International application number: (21) Application number: 98905732.8 PCT/JP98/00861 (22) Date of filing: 03.03.1998 (87) International publication number: WO 98/3931 4 (1 1 .09.1 998 Gazette 1 998/36) (84) Designated Contracting States: • MURAYAMA, Toshikazu AT BE CH DE DK ES Fl FR GB GR IE IT LI LU MC Yokkaichi-shi, Mie 51 2-091 1 (JP) NL PT SE • TSUZAKI, Nobuko Yokkaichi-shi, Mie 51 2-091 1 (JP) (30) Priority: 04.03.1997 J P 48632/97 (74) Representative: (71 ) Applicant: Kyowa Yuka Co., Ltd. Le Guen, Gerard et al Tokyo 1 00-0004 (JP) CABINET LAVOIX 2, place d'Estienne d'Orves (72) Inventors: 75441 Paris Cedex 09 (FR) • MUTO, Kenji Yokkaichi-shi, Mie551 2-091 1 (JP) (54) DIGLYCIDYL ETHERS (57) The present invention relates to a diglycidyl ether represented by the following general formula (I): R' CH?-CH-CH2-0-CH2-CH-CH2-CH-CH2-O— CH2-CH-CH2 \/ v O 0 (I) (wherein, R1 and R2 are the same or different, and represent lower alkyl having 1-6 carbon atoms), a composition including the glycidyl ether and epoxy resin, and epoxy resin cured product obtained by the composition. The glycidyl ether can be used as a reactive diluent for epoxy resins. -
G ( NOW T LSO )
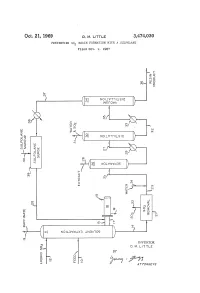
Oct. 21, 1969 D. M. E. 3,474,030 PREVENING SO RESIN FORMATION WITH A SULFOLANE Filed Oct. 9, 1967 f O O n S. NO WISO r W?? WA Q i? 1 a O r Q r C " ? l (? - 2 st e3 r g ( NOW T LSO ) SP & y (T)w 5 > < n Q - ( c O cy P O (? CO w o (f) 7: (s OW&WS ) O) O 3. R k N i 1 ? k y CO s m Yn C - CO s aa ( SS 2 Ele=E c l N 5: N NOLOW X3 NBA OS c INVENTOR. D. M. LTTLE BY y C Year A 77OAPAWAYS 3,474,030 United States Patent Office Patented Oct. 21, 1969 2 A further object of this invention is to minimize the 3,474,030 formation of resinous deposits during the operation of PREVENTING SO, RESIN FORMATION a unit employing SO2 and to provide a practical method WITH A SULFOLANE Donald M. Little, Bartlesville, Okla., assignor to Phillips for removing resinous materials inadvertently formed dur Petroleum Company, a corporation of Delaware 5 ing operation of the process. Filed Oct. 9, 1967, Ser. No. 673,607 Other objects, aspects as well as the several advantages Int. C. C10g 21/10, 21/22 of the invention will be apparent to those skilled in the U.S. C. 208-338 8 Claims art upon reading the specification, drawing, and the ap pended claims. O Summary of the invention ABSTRACT OF THE DISCLOSURE According to the invention, I have found that resinous Dissolution of resinous materials formed from SO2 and materials formed from SO2 and unsaturated materials unsaturated materials coming in contact with SO2, for ex contained in hydrocarbon oils coming in contact with SO ample, during the SO2 solvent extraction of hydrocarbon can be dissolved by contacting with at least one sulfolane. -

Supporting Information
Electronic Supplementary Material (ESI) for ChemComm. This journal is © The Royal Society of Chemistry 2015 Electronic Supplementary Information for Anodic aromatic C,C cross-coupling reaction using parallel laminar flow mode in a flow microreactor Toshihiro Arai, Hiroyuki Tateno, Koji Nakabayashi, Tsuneo Kashiwagi, and Mahito Atobe Department of Environment and System Sciences, Yokohama National University, 79-7 Tokiwadai, Hodogaya-ku, Yokohama 2408501, Japan. *To whom the correspondence should be addressed. E-mail: [email protected] This PDF file includes: Instrumentation Materials Experimental procedure Spectroscopic data S1 1. Instrumentation Nuclear magnetic resonance (1H NMR) spectra were measured on BRUKER DRX 300 1 1 spectrometer operating at 300 MHz ( H NMR) in CDCl3. All H NMR chemical shifts were reported in ppm relative to internal references of TMS at 0.00. Preparative electrolyses were carried out with a HOKUTO DENKO HABF-501A Potentiostat/Galvanostat. GCMS analyses were performed with a Shimadzu gas chromatograph mass spectrometer GCMS-QP2010. 2. Materials Acetonitrile, acetic acid, and naphthalene (1) were purchased from Kanto Chemical and used as received. Pentamethylbenzene (2), isodurene (5), mesitylene (6), 2-bromonaphthalene (8), and tetrabutylammonium tetrafluoroborate (Bu4NBF4) were purchased from Tokyo Chemical Industry and used as received. 3. Flow Microreactor Figure S1 shows construction procedure for the electrochemical two-inlet flow microreactor. The reactor was constructed from glass plates and two platinum (Pt) plates (3 cm width, 3 cm length each) (Step 1 of Figure S1). A spacer (20 m thickness double faced adhesive tape) was used to leave a rectangular channel exposed, and the two electrodes were simply sandwiched together (area of the two electrodes: 1 × 3 cm2). -

USP Excipient Reference Standards Catalog
Last Updated On: March 15, 2021 USP Excipient Reference Standards Catalog Catalog # Description Current Lot Previous CAS # NDC # Unit Price Special Restriction Lot(Valid Use Date) 1002505 Acesulfame G0H082 F0C136 (30-JUN- 55589-62-3 N/A $410.00 Potassium (200 mg) 2009) 1005706 Glacial Acetic Acid R038A0 I0M342 (31-JUL- 64-19-7 N/A $280.00 (1.5 mL/ampule; 3 2017) ampules) 1006801 Acetone (1.5 R095B0 I0M548 (28-FEB- 67-64-1 N/A $265.00 mL/ampule; 3 2022) ampules) 1009005 Acetylcysteine (200 R10200 K0K294 (30- 616-91-1 N/A $245.00 mg) NOV-2019) 1009901 Acetyltributyl Citrate R053A0 H0I337 (31-AUG- 77-90-7 N/A $275.00 (3 x 200 mg) 2017) 1009923 Acetyltriethyl Citrate R041M0 H0I339 (31-JAN- 77-89-4 N/A $275.00 (500 mg) 2017) 1012190 Adipic Acid (100 mg) F1D318 F0D318 (31- 124-04-9 N/A $265.00 MAR-2018) 1012214 Agar (500 mg) F0K137 N/A N/A $253.00 1012595 rAlbumin Human (6 R04600 G0M268 (31- N/A N/A $314.00 Cold Shipment mg) (Recombinant DEC-2016) Required Human Albumin) (COLD SHIPMENT REQUIRED) 1012688 Alcohol R05900 G0M024 (31- 64-17-5 N/A $265.00 Determination-- OCT-2018) Alcohol (5 Page 1 Last Updated On: March 15, 2021 USP Excipient Reference Standards Catalog Catalog # Description Current Lot Previous CAS # NDC # Unit Price Special Restriction Lot(Valid Use Date) mL/ampule; 5 ampules) 1012768 Alcohol (1.2 R127S0 R087W0 (31- 64-17-5 N/A $245.00 mL/ampule; 5 MAY-2020) ampules) 1012772 Dehydrated Alcohol R119J0 G0M292 (31- 64-17-5 N/A $245.00 (1.2 mL/ampule; 5 MAY-2020) ampules) 1012799 Aleuritic Acid (50 F02300 533-87-9 N/A $278.00 mg) -

Development of Sustainable Catalytic Systems for Oxidation Reactions
DOCTOR OF PHILOSOPHY Development of Sustainable Catalytic Systems For Oxidation Reactions Hughes, Nicola Award date: 2017 Awarding institution: Queen's University Belfast Link to publication Terms of use All those accessing thesis content in Queen’s University Belfast Research Portal are subject to the following terms and conditions of use • Copyright is subject to the Copyright, Designs and Patent Act 1988, or as modified by any successor legislation • Copyright and moral rights for thesis content are retained by the author and/or other copyright owners • A copy of a thesis may be downloaded for personal non-commercial research/study without the need for permission or charge • Distribution or reproduction of thesis content in any format is not permitted without the permission of the copyright holder • When citing this work, full bibliographic details should be supplied, including the author, title, awarding institution and date of thesis Take down policy A thesis can be removed from the Research Portal if there has been a breach of copyright, or a similarly robust reason. If you believe this document breaches copyright, or there is sufficient cause to take down, please contact us, citing details. Email: [email protected] Supplementary materials Where possible, we endeavour to provide supplementary materials to theses. This may include video, audio and other types of files. We endeavour to capture all content and upload as part of the Pure record for each thesis. Note, it may not be possible in all instances to convert analogue formats to usable digital formats for some supplementary materials. We exercise best efforts on our behalf and, in such instances, encourage the individual to consult the physical thesis for further information.