Space, Habitat and Isolation Are the Key Determinants of Tree Colonization by the Carpenter Ant in Plantation Forests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
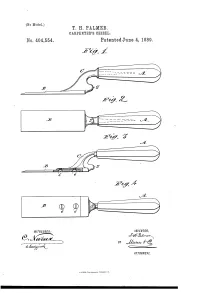
T. H. Palmer. Oarpentbb’S Chisel
(No Model.) T. H. PALMER. OARPENTBB’S CHISEL. _ No. 404,554. I PatentedJune 4, 1889. J , J WITNESSES : 4 ' l/VIg/VTOR ATTOHIVEYJ, UNITED STATES PATENT OFFICE. THE'RON H. PALMER, OF SAN BERNARDINO, CALIFORNIA. CARPENTER’S CHISEL._ SPECIFICATION forming part of Letters Patent No. 404,554, dated June 4, 1889. I Application ?led May 22, 1888.‘ Serial No. 274,724. (No model.) To all whom it may concern; 2, or it may be of a separate piece of mallea Be it known that I, THERON H. PALMER, of ble cast~ir0n or other suitable material and 40 the city and county of San Bernardino, and be joined to the blade B by screws 1) b or oth State of California, have invented a new and erwise. The shank may be secured in the. useful Improvement in Carpenters’ Chisels, handle by a simple tang or in any other de of which the following is a full, clear, and ex sired manner. The trowel-like shape of the act description. ~ > tool provides for cutting gains across a wide 45 This invention consists in a chisel or gouge surface without risk of obstruction by the ' _ for carpenters’ use which has its shank and handle, or the tool may be used as a paring handle portion bent out of line with its blade chisel or for cutting and ?tting in butts or or cutting portion and which is provided with hinges. The crooked shank O is provided at an anvil or hammer-block in rear of the blade its back, as nearly in the same plane as the to form a striking-surface when usingahani cu ttin g-blade as practicable, with aknob-like mer or mallet to force the cutting-tool up to projection S, forming a special anvil or ham its work, instead of striking on the end of mer-block to receive the blows of a hammer the handle direct, which is liable to split or bruise the handle. -

(Pentatomidae) DISSERTATION Presented
Genome Evolution During Development of Symbiosis in Extracellular Mutualists of Stink Bugs (Pentatomidae) DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Alejandro Otero-Bravo Graduate Program in Evolution, Ecology and Organismal Biology The Ohio State University 2020 Dissertation Committee: Zakee L. Sabree, Advisor Rachelle Adams Norman Johnson Laura Kubatko Copyrighted by Alejandro Otero-Bravo 2020 Abstract Nutritional symbioses between bacteria and insects are prevalent, diverse, and have allowed insects to expand their feeding strategies and niches. It has been well characterized that long-term insect-bacterial mutualisms cause genome reduction resulting in extremely small genomes, some even approaching sizes more similar to organelles than bacteria. While several symbioses have been described, each provides a limited view of a single or few stages of the process of reduction and the minority of these are of extracellular symbionts. This dissertation aims to address the knowledge gap in the genome evolution of extracellular insect symbionts using the stink bug – Pantoea system. Specifically, how do these symbionts genomes evolve and differ from their free- living or intracellular counterparts? In the introduction, we review the literature on extracellular symbionts of stink bugs and explore the characteristics of this system that make it valuable for the study of symbiosis. We find that stink bug symbiont genomes are very valuable for the study of genome evolution due not only to their biphasic lifestyle, but also to the degree of coevolution with their hosts. i In Chapter 1 we investigate one of the traits associated with genome reduction, high mutation rates, for Candidatus ‘Pantoea carbekii’ the symbiont of the economically important pest insect Halyomorpha halys, the brown marmorated stink bug, and evaluate its potential for elucidating host distribution, an analysis which has been successfully used with other intracellular symbionts. -

The Mesosomal Anatomy of Myrmecia Nigrocincta Workers and Evolutionary Transformations in Formicidae (Hymeno- Ptera)
7719 (1): – 1 2019 © Senckenberg Gesellschaft für Naturforschung, 2019. The mesosomal anatomy of Myrmecia nigrocincta workers and evolutionary transformations in Formicidae (Hymeno- ptera) Si-Pei Liu, Adrian Richter, Alexander Stoessel & Rolf Georg Beutel* Institut für Zoologie und Evolutionsforschung, Friedrich-Schiller-Universität Jena, 07743 Jena, Germany; Si-Pei Liu [[email protected]]; Adrian Richter [[email protected]]; Alexander Stößel [[email protected]]; Rolf Georg Beutel [[email protected]] — * Corresponding author Accepted on December 07, 2018. Published online at www.senckenberg.de/arthropod-systematics on May 17, 2019. Published in print on June 03, 2019. Editors in charge: Andy Sombke & Klaus-Dieter Klass. Abstract. The mesosomal skeletomuscular system of workers of Myrmecia nigrocincta was examined. A broad spectrum of methods was used, including micro-computed tomography combined with computer-based 3D reconstruction. An optimized combination of advanced techniques not only accelerates the acquisition of high quality anatomical data, but also facilitates a very detailed documentation and vi- sualization. This includes fne surface details, complex confgurations of sclerites, and also internal soft parts, for instance muscles with their precise insertion sites. Myrmeciinae have arguably retained a number of plesiomorphic mesosomal features, even though recent mo- lecular phylogenies do not place them close to the root of ants. Our mapping analyses based on previous morphological studies and recent phylogenies revealed few mesosomal apomorphies linking formicid subgroups. Only fve apomorphies were retrieved for the family, and interestingly three of them are missing in Myrmeciinae. Nevertheless, it is apparent that profound mesosomal transformations took place in the early evolution of ants, especially in the fightless workers. -

Carpenter Ants Pest D.H
58 FOREST Carpenter Ants Pest D.H. Ruppel LEAFLET Pacific Forestry Centre Fig. 1. Winged adult. Introduction tures, and forage omnivorously in the general area of the colony. Nest building activities may cause serious damage to wood in service. Their presence in houses is a nuisance. Large black ants of genus Camponotus are referred to as carpenter ants. About 20 species are known in North Ants may be discouraged or controlled with proper building 1 America, three of which are common in British Columbia. and sanitary practices. They tunnel in dead and dying trees, or in wooden struc- 1 Camponotus herculeanus modoc Wheeler (Formicidae, Hymenoptera) C. laevigatus (Smith) Wheeler (Formicidae, Hymenoptera) C. vicinus Mayr* Wheeler (Formicidae, Hymenoptera) *vicinus has a reddish thorax. It does not tunnel in wooden structures, it lives in rotting wood. Natural Resources Ressources naturelles Canada Canada Canadian Forest Service Host Carpenter ants are mainly predators, consuming a variety of insects; sugary substances are frequently sought after. They do not consume wood but only excavate nest galleries. Aphids are tended for honeydew or other exu- dations. A number of insects, most frequently a small roach, may live with ants. Description Egg: elongate, elliptical, translucent white; about 0.5 mm (1/64 inch) long. Larva: soft, legless, translucent yellow-white; varying in Fig. 2. Wingless adult. size, depending upon ultimate adult form i.e., male, female, worker; gourd-shaped body. Ants have elbowed antennae, large heads with strong Pupa: creamy-white; in ellipti- mandibles, constrictions between head and thorax and between cal, papery, light-brown thorax and abdomen. -

Effects on Brood Development in the Carpenter Ant Camponotus Vicinus Mayr After Exposure to the Yeast Associate Schwanniomyces Polymorphus Kloecker
insects Article Effects on Brood Development in the Carpenter Ant Camponotus vicinus Mayr after Exposure to the Yeast Associate Schwanniomyces polymorphus Kloecker Mark E. Mankowski 1,*, Jeffrey J. Morrell 2 and Patricia K. Lebow 3 1 Forest Products Laboratory Starkville, USDA Forest Service, Starkville, MS 39759, USA 2 Centre Timber Durability and Design Life, University of the Sunshine Coast, Sippy Downs, QLD 4102, Australia; [email protected] 3 Forest Products Laboratory Madison, USDA Forest Service, Madison, WI 53726, USA; [email protected] * Correspondence: [email protected] Simple Summary: Carpenter ants are important to ecosystem services as they assist in the breakdown of course woody debris when excavating wood for nests. Feeding on a variety of carbohydrate and protein sources, they have an infrabuccal filter that limits passage of large food particles to their gut. A variety of yeasts have been found associated with the infrabuccal pocket and the nests of these ants. The yeast Schwanniomyces polymorphus is associated with the carpenter ant Camponotus vicinus. To examine a possible nutritional association between this yeast and ant, we reared small sub-colonies of defaunated and non-defaunated C. vincus brood on several artificial diets where various nutritional components were removed. Part of the testing involved exposure of brood to these diets and cells of S. polymorphus. Dietary treatments that were augmented with yeast generally had deleterious Citation: Mankowski, M.E.; Morrell, J.J.; effects on brood development compared to diets without yeast. However, increased brood weight Lebow, P.K. Effects on Brood and increased number of adult ants from initial brood was observed in non-defaunated ants fed a Development in the Carpenter Ant diet where B vitamins and sterols were absent, but augmented with live yeast. -

Fecundity of Ant Queens in Relation to Their Age and the Mode of Colony Founding L
Insectes Sociaux, Paris Masson, Paris, 1990 1990, Volume 37, n ~ 2, pp. 116-130 FECUNDITY OF ANT QUEENS IN RELATION TO THEIR AGE AND THE MODE OF COLONY FOUNDING L. KELLER (1) and L. PASSERA (2) (1) Musde Zoologique, Palais de Rumine, CP 448, 1000 Lausanne 17, Switzerland (2) Laboratoire d'Entomologie, Universitd Paul Sabatier, 118, route de Narbonne, F 31062 Toulouse Cedex, France, U.A. C.N.R.S. 303 Regu le 23 janvier 1989 Accept6 le 15 juin 1989 SUMMARY The change over time in the fecundity and weight of queens was investigated in three monogynous, independent colony founding species, Lasius niger, Camponotus ligniperda and C. herculaneus, and two polygynous dependent colony founding species, Plagiolepis pygmaea and Iridomyrmex humilis. Queens of the three species founding independently exhibited a similar pattern with a significant loss of weight between mating and the emergence of the first workers. In contrast, weights of queens of the species employing dependent colony founding remained more stable. Fecundity of queens founding inde- pendently increased slowly with time whereas fecundity of queens founding dependently reached the maximum level some weeks after the beginning of the first reproductive season. These results are discussed in relation to some differences in the life history (e.g., life-span) between queens utilizing independent and dependent colony founding. RESUME Fdcondit6 des reines de fourmis en relation avec leur &ge et le mode de fondation de la soci6t6 On a 6tud6 dans ce travail les variations en fonction du temps de la f6condit6 et du poids des reines fondatrices de trois esp6ces monogynes h fondation ind6pendante (Lasius niger, Camponotus ligniperda, Camponotus herculeanus) et de deux esp6ces polygynes h fondation d6pendante (Plagiolepis pygmaea et Iridomyrmex humilis). -

Nutritional Ecology of the Carpenter Ant Camponotus Pennsylvanicus (De Geer): Macronutrient Preference and Particle Consumption
Nutritional Ecology of the Carpenter Ant Camponotus pennsylvanicus (De Geer): Macronutrient Preference and Particle Consumption Colleen A. Cannon Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Entomology Richard D. Fell, Chairman Jeffrey R. Bloomquist Richard E. Keyel Charles Kugler Donald E. Mullins June 12, 1998 Blacksburg, Virginia Keywords: diet, feeding behavior, food, foraging, Formicidae Copyright 1998, Colleen A. Cannon Nutritional Ecology of the Carpenter Ant Camponotus pennsylvanicus (De Geer): Macronutrient Preference and Particle Consumption Colleen A. Cannon (ABSTRACT) The nutritional ecology of the black carpenter ant, Camponotus pennsylvanicus (De Geer) was investigated by examining macronutrient preference and particle consumption in foraging workers. The crops of foragers collected in the field were analyzed for macronutrient content at two-week intervals through the active season. Choice tests were conducted at similar intervals during the active season to determine preference within and between macronutrient groups. Isolated individuals and small social groups were fed fluorescent microspheres in the laboratory to establish the fate of particles ingested by workers of both castes. Under natural conditions, foragers chiefly collected carbohydrate and nitrogenous material. Carbohydrate predominated in the crop and consisted largely of simple sugars. A small amount of glycogen was present. Carbohydrate levels did not vary with time. Lipid levels in the crop were quite low. The level of nitrogen compounds in the crop was approximately half that of carbohydrate, and exhibited seasonal dependence. Peaks in nitrogen foraging occurred in June and September, months associated with the completion of brood rearing in Camponotus. -

Invertebrates of the Macocha Abyss (Moravian Karst, Czech Republic) Nevretenčarji Brezna Macoha (Moravski Kras, Republika Češka)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ZRC SAZU Publishing (Znanstvenoraziskovalni center - Slovenske akademije znanosti... COBISS: 1.02 INVERTEBRATES OF THE MACOCHA ABYSS (MORAVIAN KARST, CZECH REPUBLIC) NEVRETENČARJI BREZNA MACOHA (MORAVSKI KRAS, REPUBLIKA ČEŠKA) Vlastimil RŮŽIČKA1, Roman MLEJNEK2, Lucie JUŘIČKOVÁ3, Karel TAJOVSKÝ4, Petr ŠMILAUER5 & Petr ZAJÍČEK2 Abstract UDC 592:551.44(437.32) Izvleček UDK 592:551.44(437.32) Vlastimil Růžička, Roman Mlejnek, Lucie Juřičková, Karel Vlastimil Růžička, Roman Mlejnek, Lucie Juřičková, Karel Tajovský, Petr Šmilauer & Petr Zajíček: Invertebrates of the Tajovský, Petr Šmilauer & Petr Zajíček: Nevretenčarji brezna Macocha Abyss (Moravian Karst, Czech Republic) Macoha (Moravski kras, Republika Češka) The invertebrates of the Macocha Abyss, Moravian Karst, Med vzorčenjem v letih 2007 in 2008 smo v jami Maco- Czech Republic, were collected in 2007–2008 and 222 species ha določili 222 vrst nevretenčarjev. Ovrednotili smo rela- were identified in total. The relative abundance of individual tivno pogostost posameznih taksonov polžev, suhih južin, taxa of land snails, harvestmen, pseudoscorpions, spiders, mil- paščipalcev, pajkov, stonog, kopenskih enakonožcev, hroščev lipedes, centipedes, terrestrial isopods, beetles, and ants was in mravelj. Na mraz prilagojene gorske in podzemeljske vrste evaluated. The cold-adapted mountain and subterranean spe- naseljujejo dno in spodnji del brezna, toploljubne vrste pa cies inhabit the bottom and lower part of the abyss, whereas naseljujejo kamnite površine soncu izpostavljenega roba. V the sun-exposed rocky margins were inhabited by thermophil- Macohi je več ogroženih vrst, ki jih sicer v okoliški pokrajini ne ous species. Macocha harbors several threatened species that najdemo. Kot habitat s specifično mikroklimo je Macoha izje- are absent or very rare in the surrounding habitats. -

Bericht Der Entomolo- Gischen Kartierung 2010)
Entomologische Kartierung in der „Pfarrwiese“ und in einem Sandabbruch in Hofstätten Spinnen, Heuschrecken, Wanzen, Zikaden, Tagfalter & Widderchen, Käfer, Ameisen (Araneae, Saltatoria, Heteroptera, Auchenorrhyncha, Diurna & Zygaenidae, Coleoptera, Formicidae) Europaschutzgebiet: „Teile des Südoststeirischen Hügellandes inklusive Grabenlandbäche und Höll“ Im Auftrag von: Verein Lebende Erde im Vulkanland (L.E.i.V.), Bernard Wieser Bearbeitung: Thomas Frieß, Erwin Holzer, Anton Koschuh, Gernot Kunz, Alexander Platz, Herbert Wagner Graz, im Juni 2011 Entomologische Kartierung 2010 / Hofstätten L.E.i.V. Inhaltsverzeichnis 1 Einleitung und Fragestellungen ....................................................................................4 2 Untersuchte Standorte ...................................................................................................5 3 Ergebnisse und Diskussion .........................................................................................10 3.1 Spinnen (Araneae)................................................................................................10 3.1.1 Methodik .............................................................................................................10 3.1.2 Artenliste.............................................................................................................11 3.1.3 Kommentare zu ausgewählten Arten..................................................................13 3.1.4 Faunistische, zönotische und naturschutzfachliche Aspekte..............................14 -

Easychair Preprint Ant Microbial Endosymbionts and the Emergent
EasyChair Preprint № 1661 Ant microbial endosymbionts and the emergent properties of social groups Alessio Sclocco, Shirlyn Jia Yun Ong and Serafino Teseo EasyChair preprints are intended for rapid dissemination of research results and are integrated with the rest of EasyChair. October 14, 2019 Ant microbial endosymbionts and the emergent properties of social groups Alessio Sclocco1, Shirlyn Jia Yun Ong2 and Serafino Teseo2† 1Netherlands eScience Center, Amsterdam, The Netherlands (E-mail: [email protected]) 2School of Biological Sciences, Nanyang Technological University, Singapore (Tel: +65 63167088; E-mail: [email protected]) Abstract: In the last fifteen years, research on animal models has provided advances on how gut symbiotic microbes affect behavior and its underlying neurophysiology. However, most studies on the gut microbiota only take into exam individual behavior without considering social dynamics. Contrarily, animals and humans live in complex societies where they constantly adjust physiology and behavior to social interactions. To improve our understanding of how microbes and hosts interact and produce functional individual, social and collective phenotypes, we need to broaden our experimental approaches to a group-level dimension. The ideal models for this purpose are social animals living in stable symbioses with microbes, such as eusocial insects. In our research, we investigate Camponotus carpenter ants and their obligate bacterial symbiont Blochmannia from a behavioral ecology perspective. We aim to create ant colonies including differential proportions of bacteria-free individuals by suppressing Blochmannia with antibiotics. Then, using a machine learning-based video tracking system, we will study network features and group-level behavior of such experimental colonies. Keywords: Social Evolution; Microbiota-Gut-Brain Axis; Ants; Group-Level Behavior; Machine Learning; Real Time Data Analysis 1. -

Table S4. Phylogenetic Distribution of Bacterial and Archaea Genomes in Groups A, B, C, D, and X
Table S4. Phylogenetic distribution of bacterial and archaea genomes in groups A, B, C, D, and X. Group A a: Total number of genomes in the taxon b: Number of group A genomes in the taxon c: Percentage of group A genomes in the taxon a b c cellular organisms 5007 2974 59.4 |__ Bacteria 4769 2935 61.5 | |__ Proteobacteria 1854 1570 84.7 | | |__ Gammaproteobacteria 711 631 88.7 | | | |__ Enterobacterales 112 97 86.6 | | | | |__ Enterobacteriaceae 41 32 78.0 | | | | | |__ unclassified Enterobacteriaceae 13 7 53.8 | | | | |__ Erwiniaceae 30 28 93.3 | | | | | |__ Erwinia 10 10 100.0 | | | | | |__ Buchnera 8 8 100.0 | | | | | | |__ Buchnera aphidicola 8 8 100.0 | | | | | |__ Pantoea 8 8 100.0 | | | | |__ Yersiniaceae 14 14 100.0 | | | | | |__ Serratia 8 8 100.0 | | | | |__ Morganellaceae 13 10 76.9 | | | | |__ Pectobacteriaceae 8 8 100.0 | | | |__ Alteromonadales 94 94 100.0 | | | | |__ Alteromonadaceae 34 34 100.0 | | | | | |__ Marinobacter 12 12 100.0 | | | | |__ Shewanellaceae 17 17 100.0 | | | | | |__ Shewanella 17 17 100.0 | | | | |__ Pseudoalteromonadaceae 16 16 100.0 | | | | | |__ Pseudoalteromonas 15 15 100.0 | | | | |__ Idiomarinaceae 9 9 100.0 | | | | | |__ Idiomarina 9 9 100.0 | | | | |__ Colwelliaceae 6 6 100.0 | | | |__ Pseudomonadales 81 81 100.0 | | | | |__ Moraxellaceae 41 41 100.0 | | | | | |__ Acinetobacter 25 25 100.0 | | | | | |__ Psychrobacter 8 8 100.0 | | | | | |__ Moraxella 6 6 100.0 | | | | |__ Pseudomonadaceae 40 40 100.0 | | | | | |__ Pseudomonas 38 38 100.0 | | | |__ Oceanospirillales 73 72 98.6 | | | | |__ Oceanospirillaceae -

The Functions and Evolution of Social Fluid Exchange in Ant Colonies (Hymenoptera: Formicidae) Marie-Pierre Meurville & Adria C
ISSN 1997-3500 Myrmecological News myrmecologicalnews.org Myrmecol. News 31: 1-30 doi: 10.25849/myrmecol.news_031:001 13 January 2021 Review Article Trophallaxis: the functions and evolution of social fluid exchange in ant colonies (Hymenoptera: Formicidae) Marie-Pierre Meurville & Adria C. LeBoeuf Abstract Trophallaxis is a complex social fluid exchange emblematic of social insects and of ants in particular. Trophallaxis behaviors are present in approximately half of all ant genera, distributed over 11 subfamilies. Across biological life, intra- and inter-species exchanged fluids tend to occur in only the most fitness-relevant behavioral contexts, typically transmitting endogenously produced molecules adapted to exert influence on the receiver’s physiology or behavior. Despite this, many aspects of trophallaxis remain poorly understood, such as the prevalence of the different forms of trophallaxis, the components transmitted, their roles in colony physiology and how these behaviors have evolved. With this review, we define the forms of trophallaxis observed in ants and bring together current knowledge on the mechanics of trophallaxis, the contents of the fluids transmitted, the contexts in which trophallaxis occurs and the roles these behaviors play in colony life. We identify six contexts where trophallaxis occurs: nourishment, short- and long-term decision making, immune defense, social maintenance, aggression, and inoculation and maintenance of the gut microbiota. Though many ideas have been put forth on the evolution of trophallaxis, our analyses support the idea that stomodeal trophallaxis has become a fixed aspect of colony life primarily in species that drink liquid food and, further, that the adoption of this behavior was key for some lineages in establishing ecological dominance.