The Endocrine System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -
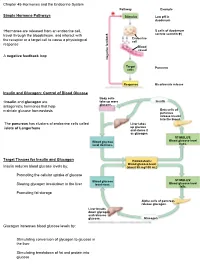
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Human Microbiome: Your Body Is an Ecosystem
Human Microbiome: Your Body Is an Ecosystem This StepRead is based on an article provided by the American Museum of Natural History. What Is an Ecosystem? An ecosystem is a community of living things. The living things in an ecosystem interact with each other and with the non-living things around them. One example of an ecosystem is a forest. Every forest has a mix of living things, like plants and animals, and non-living things, like air, sunlight, rocks, and water. The mix of living and non-living things in each forest is unique. It is different from the mix of living and non-living things in any other ecosystem. You Are an Ecosystem The human body is also an ecosystem. There are trillions tiny organisms living in and on it. These organisms are known as microbes and include bacteria, viruses, and fungi. There are more of them living on just your skin right now than there are people on Earth. And there are a thousand times more than that in your gut! All the microbes in and on the human body form communities. The human body is an ecosystem. It is home to trillions of microbes. These communities are part of the ecosystem of the human Photo Credit: Gaby D’Alessandro/AMNH body. Together, all of these communities are known as the human microbiome. No two human microbiomes are the same. Because of this, you are a unique ecosystem. There is no other ecosystem like your body. Humans & Microbes Microbes have been around for more than 3.5 billion years. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Neuroscience
NEUROSCIENCE SCIENCE OF THE BRAIN AN INTRODUCTION FOR YOUNG STUDENTS British Neuroscience Association European Dana Alliance for the Brain Neuroscience: the Science of the Brain 1 The Nervous System P2 2 Neurons and the Action Potential P4 3 Chemical Messengers P7 4 Drugs and the Brain P9 5 Touch and Pain P11 6 Vision P14 Inside our heads, weighing about 1.5 kg, is an astonishing living organ consisting of 7 Movement P19 billions of tiny cells. It enables us to sense the world around us, to think and to talk. The human brain is the most complex organ of the body, and arguably the most 8 The Developing P22 complex thing on earth. This booklet is an introduction for young students. Nervous System In this booklet, we describe what we know about how the brain works and how much 9 Dyslexia P25 there still is to learn. Its study involves scientists and medical doctors from many disciplines, ranging from molecular biology through to experimental psychology, as well as the disciplines of anatomy, physiology and pharmacology. Their shared 10 Plasticity P27 interest has led to a new discipline called neuroscience - the science of the brain. 11 Learning and Memory P30 The brain described in our booklet can do a lot but not everything. It has nerve cells - its building blocks - and these are connected together in networks. These 12 Stress P35 networks are in a constant state of electrical and chemical activity. The brain we describe can see and feel. It can sense pain and its chemical tricks help control the uncomfortable effects of pain. -

Biology F – Lesson 4 – Endocrine & Reproductive Systems
Unit: Biology F – Endocrine & Reproduction LESSON 4.1 - AN INTRODUCTION TO THE ENDOCRINE SYSTEM Overview: Students research the location and function of the major endocrine glands and consider some endocrine system disorders. Suggested Timeline: 1 hour Materials: • An Introduction to the Endocrine System (Student Handout) • QUIZ – The Endocrine System (Student Handout) • student access to computers with the internet Method: 1. Allow students to use computers with internet access and other resources from the library to complete their questions on the endocrine system. 2. Students write quiz on the endocrine system. Assessment and Evaluation: • Affective assessment of student understanding of parts of the endocrine system • Student grade on quiz Extension: Assign a student or group of students to ‘be’ a specific endocrine gland. Have them role play for the class to get the idea of the function of the gland across to their classmates. Science 21 Bio F – Endocrine B152 Unit: Biology F – Endocrine & Reproduction Student Handout Name: ______________ Partner(s): _________________________ Date: ____________ Period: _____ An Introduction to the Endocrine System Go to the following website: http://health.howstuffworks.com/adam-200091.htm Watch the video to complete the following questions. 1. The endocrine system is composed primarily of _________________ that produce chemical messengers called __________________. 2. List the names of glands of the endocrine system, as mentioned in the video. Hint: There are 8 in total. 3. The endocrine and ________________ systems work closely together. The brain sends info to the endocrine glands and the endocrine glands send feedback to the brain. 4. The part of the brain that is known as the ‘master switchboard’ and controls the endocrine system is called the _____________________. -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -

Chapter 20: Endocrine System
EndocrineEndocrine SystemSystem Modified by M. Myers 1 TheThe EndocrineEndocrine SystemSystem 2 EndocrineEndocrine GlandsGlands z The endocrine system is made of glands & tissues that secrete hormones. z Hormones are chemicals messengers influencing a. metabolism of cells b. growth and development c. reproduction, d. homeostasis. 3 HormonesHormones Hormones (chemical messengers) secreted into the bloodstream and transported by blood to specific cells (target cells) Hormones are classified as 1. proteins (peptides) 2. Steroids 4 HormoneHormone ClassificationClassification z Steroid Hormones: – Lipid soluble – Diffuse through cell membranes – Endocrine organs z Adrenal cortex z Ovaries z Testes z placenta 5 HormoneHormone ClassificationClassification z Nonsteroid Hormones: – Not lipid soluble – Received by receptors external to the cell membrane – Endocrine organs z Thyroid gland z Parathyroid gland z Adrenal medulla z Pituitary gland z pancreas 6 HormoneHormone ActionsActions z “Lock and Key” approach: describes the interaction between the hormone and its specific receptor. – Receptors for nonsteroid hormones are located on the cell membrane – Receptors for steroid hormones are found in the cell’s cytoplasm or in its nucleus 7 http://www.wisc- online.com/objects/index_tj.asp?objID=AP13704 8 EndocrineEndocrine SystemSystem z There is a close assoc. b/w the endocrine & nervous systems. z Hormone secretion is usually controlled by either negative feedback or antagonistic hormones that oppose each other’s actions 9 HypothalamusHypothalamus 1. regulates the internal environment through the autonomic system 2. controls the secretions of the pituitary gland. 10 HypothalamusHypothalamus && PituitaryPituitary GlandGland posteriorposterior pituitary/pituitary/ anterioranterior pituitarypituitary 11 PosteriorPosterior PituitaryPituitary The posterior pituitary secretes zantidiuretic hormone (ADH) zoxytocin 12 13 14 AnteriorAnterior pituitarypituitary glandgland 1. -

The Human Body Is Like a Complex Machine, with Many Little Parts That Work by Themselves Or with Other Parts to Perform Specific Functions
Have you ever wondered about how the human body works? The human body is like a complex machine, with many little parts that work by themselves or with other parts to perform specific functions. Sometimes, it seems like our body has a mind of its own and it embarrasses you. Have you ever passed gas accidentally when other people were around? In most cases, the things that your body does are normal, but it’s important to know your body so you can recognize what is normal body behavior and what is not. If there is a problem with how your body works, by recognizing that there is a problem, you can take steps to fix it or get help. To understand how the body works, it helps to understand how the body is organized. The smallest living unit in any organism is a cell and the human body is made up of trillions of them. That is more than 1,000,000,000,000 cells! Cells are so small you cannot see them without a microscope. Cells are important for many reasons. They produce the energy in your body to do daily activities, or hold the coded instructions for everything from the color of your hair to whether you have freckles or not. Cells differentiate from each other to perform different, important tasks within the body. For example, some cells might become brain cells while others make bone, and red blood cells carry oxygen throughout the body, while white blood cells fight infection. When a group of cells work together to perform ?a specific function, they are called tissue. -

Nanos in the Human Body – Medical Perspectives and Ethical Concerns
36 Autumn-winter 2009/HesaMag #01 Special report 25/30 Nanos in the human body – Medical perspectives and ethical concerns Healthcare is a priority focus of nanotechnology research, where the convergence of nanosciences, molecular and cell biology, and medicine can act to deliver improvements in human health and quality of life. It is an appealing prospect; but as with any new technology, there are both ethical and health and safety issues to be addressed, especially where applications in human bodies are concerned. Aída Maria Ponce Del Castillo ETUI Researcher Nanotechnologies can be seen as a Other developments are the generation “tool-kit” that enables different life sciences of nanomaterials that could be applied to im- to work together to produce new tools for di- prove tissue regeneration, like restoring car- agnosis and treatments. tilage function to overcome arthritis, in vitro Advances in nanotechnology harbour engineered organ patches or biomaterials for many potential uses in medicine. They will in situ regeneration of bones. Nanotechnol- help increase our understanding of the hu- ogy could also help in the fight against cancer, 1. National Nanotechnology man body, its mechanisms and diseases, and by developing nanomaterial systems to attack Initiative 2003, how to restore it to health. and destroy tumours. Workshop Report on One possible application will be to cus- Their multiple properties give nanoma- Nanobiotechnology, tomise medical treatment through personal- terials a wide spectrum of medical uses; some Virginia, p. 39. Liposomes for drug delivery ised medicine delivery, where patients will have a biocidal activity used in self-cleaning in some cases of cancer be given the precise, controlled dose of their surfaces. -

Grade 6: the Heart and Circulatory System Lesson 1: the Heart Lesson 2: the Heart Rate Lesson 3: the Circulatory System and Blood
Grade 6: The Heart and Circulatory System Lesson 1: The Heart Lesson 2: The Heart Rate Lesson 3: The Circulatory System and Blood Objectives: 1. Students will identify the four chambers of the heart 2. Students will identify four important structures of the Circulatory System and what they do. 3. Students will explain heart rate and be able to take their resting and active heart rates. 4. Students will describe the major functions of the Circulatory System. 5. Students will explain the role of the heart in circulation 6. Students will give a basic explanation of the cardio-pulmonary sequence. 7. Students will describe systemic circulation. Materials: Lesson 1: • Animal heart (Example: cow, pig, sheep) • Note cards • Picture of the heart (See Figure 1) • Dissection tools (Scissors, pan, etc.) Lesson 2: • Small drum • Watch or clock with second hand, or time • Optional: Stethoscope Lesson 3: • Corn Syrup • Plastic Beads: flat red disks, white ovals, green or blue seed beads • “Explain” experiment (per group): o Two small balloons or large finger cots o One clear tube (1/2” diameter) about 8” long o One clear tube (3/4” diameter) about 8” long o 16 - 20 oz. water o Red food coloring o Measuring cup o Funnel o Two empty plastic containers (such as cottage cheese or yogurt cartons) Activity Summary: In this lesson students will learn the basic functioning of the heart, Circulatory System and blood, the connection to lung functioning, and the activity of the Grade 6: The Heart & Circulatory System – Revised 2008 Page 1 Circulatory System in the body. -

SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This
12/19/2018 11:00 AM FOUNDATIONAL LEARNING SYSTEM 092892-181219 © Johnson & Johnson Servicesv Inc. 2018 All rights reserved. 1 SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This module will strengthen your understanding of basic neuroanatomy, neurovasculature, and functional roles of specific brain regions. 1 12/19/2018 11:00 AM Lesson 1: Introduction to the Brain The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. In this lesson, we’ll discuss the principle brain regions, layers of the brain, and lobes of the brain, as well as common terms used to orient neuroanatomical discussions. 2 SAY: The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. (Purves 2012/p717/para1) In this lesson, we’ll explore these organizational layers by discussing the principle brain regions, layers of the brain, and lobes of the brain. We’ll also discuss the terms used by scientists and healthcare providers to orient neuroanatomical discussions. 2 12/19/2018 11:00 AM Lesson 1: Learning Objectives • Define terms used to specify neuroanatomical locations • Recall the 4 principle regions of the brain • Identify the 3 layers of the brain and their relative location • Match each of the 4 lobes of the brain with their respective functions 3 SAY: Please take a moment to review the learning objectives for this lesson. 3 12/19/2018 11:00 AM Directional Terms Used in Anatomy 4 SAY: Specific directional terms are used when specifying the location of a structure or area of the brain.