Causes of Mortality Among Whistling Ducks in Captivity NIGELLA HILLGARTH and JANET KEAR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Black-Bellied Whistling-Duck Yellow-Billed Cuckoo Fulvous
Black-bellied Whistling-Duck Yellow-billed Cuckoo Willet* Band-rumped Storm-Petrel Northern Flicker Bewick's Wren Fulvous Whistling-Duck Black-billed Cuckoo Greater Yellowlegs Wood Stork Pileated Woodpecker Blue-gray Gnatcatcher Snow Goose* Common Nighthawk Wilson's Phalarope Magnificent Frigatebird American Kestrel Golden-crowned Kinglet Ross's Goose Chuck-will's-widow Red-necked Phalarope Northern Gannet Merlin* Ruby-crowned Kinglet Greater White-fronted Goose Eastern Whip-poor-will Red Phalarope Anhinga Peregrine Falcon Eastern Bluebird Brant Chimney Swift Great Skua Great Cormorant Monk Parakeet Veery Cackling Goose Ruby-throated Hummingbird Pomarine Jaeger Double-crested Cormorant Ash-throated Flycatcher Gray-cheeked Thrush Canada Goose Rufous Hummingbird Parasitic Jaeger American White Pelican Great Crested Flycatcher Bicknell's Thrush Mute Swan Clapper Rail Long-tailed Jaeger Brown Pelican Western Kingbird Swainson's Thrush Trumpeter Swan King Rail Dovekie American Bittern Eastern Kingbird Hermit Thrush Tundra Swan Virginia Rail Thick-billed Murre Least Bittern Gray Kingbird Wood Thrush Wood Duck Sora Razorbill Great Blue Heron* Scissor-tailed Flycatcher American Robin Atlantic Puffin Great Egret Blue-winged Teal Common Gallinule Fork-tailed Flycatcher Varied Thrush Black-legged Kittiwake Snowy Egret Northern Shoveler American Coot Olive-sided Flycatcher Gray Catbird Sabine's Gull Little Blue Heron Gadwall Purple Gallinule Eastern Wood-Pewee Brown Thrasher Bonaparte's Gull Tricolored Heron Eurasian Wigeon Yellow Rail Yellow-bellied -

Birds of Bharatpur – Check List
BIRDS OF BHARATPUR – CHECK LIST Family PHASIANIDAE: Pheasants, Partridges, Quail Check List BLACK FRANCOLIN GREY FRANCOLIN COMMON QUAIL RAIN QUAIL JUNGLE BUSH QUAIL YELLOW-LEGGED BUTTON QUAIL BARRED BUTTON QUAIL PAINTED SPURFOWL INDIAN PEAFOWL Family ANATIDAE: Ducks, Geese, Swans GREATER WHITE-FRONTED GOOSE GREYLAG GOOSE BAR-HEADED GOOSE LWSSER WHISTLING-DUCK RUDDY SHELDUCK COMMON SHELDUCK COMB DUCK COTTON PYGMY GOOSE MARBLED DUCK GADWALL FALCATED DUCK EURASIAN WIGEON MALLARD SPOT-BILLED DUCK COMMON TEAL GARGANEY NORTHERN PINTAIL NORTHERN SHOVELER RED-CRESTED POCHARD COMMON POCHARD FERRUGINOUS POCHARD TUFTED DUCK BAIKAL TEAL GREATER SCAUP BAER’S POCHARD Family PICIDAE: Woodpeckers EURASIAN WRYNECK BROWN-CAPPED PYGMY WOODPECKER YELLOW-CROWNED WOODPECKER BLACK-RUMPED FLAMBACK Family CAPITONIDAE: Barbets BROWN-HEADED BARBET COPPERSMITH BARBET Family UPUPIDAE: Hoopoes COMMON HOOPOE Family BUCEROTIDAE: Hornbills INDAIN GREY HORNBILL Family CORACIIDAE: Rollers or Blue Jays EUROPEAN ROLLER INDIAN ROLLER Family ALCEDINIDAE: Kingfisher COMMON KINGFISHER STORK-BILLED KINGFISHER WHITE-THROATED KINGFISHER BLACK-CAPPED KINGFISHER PIED KINGFISHER Family MEROPIDAE: Bee-eaters GREEN BEE-EATER BLUE-CHEEKED BEE-EATER BLUE-TAILED BEE-EATER Family CUCULIDAE: Cuckoos, Crow-pheasants PIED CUCKOO CHESTNUT-WINGED CUCKOO COMMON HAWK CUCKOO INDIAN CUCKOO EURASIAN CUCKOO GREY-BELLIED CUCKOO PLAINTIVE CUCKOO DRONGO CUCKOO ASIAN KOEL SIRKEER MALKOHA GREATER COUCAL LESSER COUCAL Family PSITTACIDAS: Parrots ROSE-RINGED PARAKEET PLUM-HEADED PARKEET Family APODIDAE: -

Salvadori's Duck of New Guinea
Salvadori’s Duck of New Guinea J . K E A R When Phillips published the fourth volume of to the diving ducks and mergansers. Then his Natural History of the Ducks in 1926, he Mayr (1931a) was able to examine a dead noted that Salvadori’s Duck Salvadorina specimen and found that the sternum and (Anas) waigiuensis was ‘the last to be brought trachea of the male were, although smaller, to light and perhaps the most interesting of the similar to those of the Mallard Anas platy peculiar anatine birds of the world’. It was still rhynchos. As a result, Salvadori’s Duck was practically unknown in 1926; the first moved to the genus A nas. However, as recorded wild nest was not seen until 1959 (H. Niethammer (1952) and Kear (1972) later M. van Deusen, in litt.), and even today the pointed out, the trachea of the Torrent and species has been little studied. Blue Duck are also Anas-like, so that, on this The first specimen was called Salvadorina character, the genera M e rg a n e tta and waigiuensis in 1894 by the Hon. Walter Hymenolaimus could also be eliminated, and Rothschild and by Dr Ernst Hartert, his all three species put with the typical dabbling curator at the Zoological Museum at Tring. ducks. The name was to honour Count Tomasso The present author had already studied the Salvadori, the Italian taxonomist who Blue Duck in New Zealand (Kear, 1972), and specialized both in waterfowl and in the birds was lucky enough to be able to watch of Papua New Guinea. -

ILSOLC Bird Checklist
Birding in Seguin Irma Lewis Seguin Outdoor Irma Lewis Seguin, Texas is located in south- central Texas, in an ecological area on Learning Center Seguin Outdoor Learning the boundary of Blackland Prairie to the north and the Post Oak Savannah The Seguin Outdoor Learning Center to the south and east. Most of the Center a 115-acre private, non surrounding land is in agricultural use, primarily cattle grazing, providing a -profit educational facility fairly diverse environment for birds. nestled along Geronimo Creek The Guadalupe River runs through the in northeast Seguin. Our city. Large pecan and cypress trees line the river, including the city park, facilities include a pavilion, Starcke Park, on Bus. 123 South. The natural history center, walking trail in Starcke Park East, along the confluence of Walnut Branch, environmental science center, offers good birding for warblers, blue- amphitheater, ropes course, “Education Through Experience For All Ages” birds and other passerines. Several small reservoirs located along the river nature trail, outdoor class- near town, including Lakes Dunlap, room and pond. Schools, youth McQueeney, and Placid also provide groups, sports teams, clubs, areas for waterfowl. churches and corporations enjoy our peaceful, natural Some species that are common around setting where children and Seguin may be of special interest to citizens of the community can birders from other regions. learn through discovery and Scissor-tailed Flycatchers are unique adventure common during the breeding season. Look for them on fences and telephone experiences. wires anywhere in the countryside around Seguin. Crested Caracaras are The ILSOLC is open to also common in the countryside and are Birding Hours: members, scheduled and especially visible when feeding on Monday-Friday, 8a-5p road-kill carcasses, often in the supervised groups only. -

A 2010 Supplement to Ducks, Geese, and Swans of the World
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 20. https://digitalcommons.unl.edu/biosciducksgeeseswans/20 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The World’s Waterfowl in the 21st Century: A 200 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard Pages xvii–xxiii: recent taxonomic changes, I have revised sev- Introduction to the Family Anatidae eral of the range maps to conform with more current information. For these updates I have Since the 978 publication of my Ducks, Geese relied largely on Kear (2005). and Swans of the World hundreds if not thou- Other important waterfowl books published sands of publications on the Anatidae have since 978 and covering the entire waterfowl appeared, making a comprehensive literature family include an identification guide to the supplement and text updating impossible. -

Reese--Waterfowl Presentation
Waterfowl – What they are and are not Great variety in sizes, shapes, colors All have webbed feet and bills Sibley reading is great for lots of facts and insights into the group Order Anseriformes Family Anatidae – 154 species worldwide, 44 in NA Subfamily Dendrocygninae – whistling ducks Subfamily Anserinae – geese & swans Subfamily Anatinae - ducks Handout • Indicates those species occurring regularly in ID Ducks = ?? Handout • Indicates those species occurring regularly in ID Ducks = 26 species Geese = ?? Handout • Indicates those species occurring regularly in ID Ducks = 26 species Geese = 6 (3 are rare) Swans = ?? Handout • Indicates those species occurring regularly in ID Ducks = 26 species Geese = 6 Swans = 2 What separates the 3 groups? Ducks - Geese - Swan - What separates the 3 groups? Ducks – smaller, shorter legs & necks Geese – long legs, graze in uplands Swan – shorter legs than geese, long necks, larger size, feed more often in water Non-waterfowl Cranes, rails – beak, rails have lobed feet Coots – bill but lobed feet Grebes – lobed feet Loons – webbed feet but sharp beak Woodcock and snipe – feet not webbed, beaks These are not on handout but are managed by USFWS as are waterfowl On handout: Subfamily Dendrocygninae – whistling ducks – used to be called tree ducks 2 species, more goose-like, longer necks and legs than most ducks, flight is faster than geese, but slower than other ducks Occur along gulf coast and Florida On handout: Subfamily Anserinae – geese and swans Tribe Cygnini – swans – 2 species Tribe Anserini – -

Waterfowl Collection at Slimbridge 1955-56
Annual Report 1954-56 35 WATERFOWL COLLECTION AT SLIMBRIDGE 1955-56 THE BREEDING SEASON 1955 By S. T. Johnstone T h e feature of the breeding season was the striking effect of cold weather on the well-being of the young birds. Frost in February and March may well have reduced considerably the hatchability of the Ne-Ne eggs, all 31 of which were laid during a period when the cold was so extreme that some African Black Duck eggs were split open before they could be collected. The very wet April and May caused flooding of nests and indeed several sitting boxes suffered in this way. This latter occurrence may have had a bearing on the unfortunate rise in the incidence of Aspergillosis. Pathogenic mould was found in a number of fertile eggs that failed to hatch and a relatively large number of goslings succumbed to mycosis. In 1956 the use of sawdust for nest making in the sitting boxes has been discontinued in favour of peat moss impregnated with a fungicide. The two pumping systems installed in the spring of 1954 have enabled us to provide relatively fast-flowing water through the rearing pens. By this means we have get rid of the concentration of water fleas (.Daphnia pulex). This had been the host of Acuaria uncinata, a worm inhabiting the proventriculus and causing wasting and subsequent death. We are pleased to report that not a single case of Acuaria was recorded in 1955. It was a great relief to those concerned with the rearing when cold and wet ceased and the long warm sunny days of June and July appeared as a panacea to all ills save the losses from predators. -

Population Management of Anseriformes in AZA Keith Lovett
Population Management of Anseriformes in AZA Keith Lovett Executive Director of Buttonwood Park Zoo and Buttonwood Park Zoological Society Anseriformes Taxon Advisory Group Chair Waterfowl Diversity in AZA 135 SPECIES Waterfowl Declines in AZA Why the Decline? Loss of Knowledge Loss of Waterfowl Exhibits New Exhibit Design New Exhibit Design New Exhibit Design Cost Acquisition Quarantine Shipment Exams Cost Examples Acquisition: $250 Shipment by commercial airline: $150 Quarantine medical testing (CBC, Chem Profile, Fecal Culture): $300 (2 birds) Vet Care Labor: $500 (1 hr a day for 30 days plus exit exam) Total: $1200 Zoos and Private Aviculture How do we respond? Managed Programs List of SSP’s GREEN SSP – NONE YELLOW SSP – 10 RED SSP – 3 CANDIDATE PROGRAM - 2 Criteria Yellow SSP White-wing Wood Duck - 48.55.8 (111) at 13 institutions African Pygmy Goose - 50.45.3 (98) at 24 institutions Swan Goose – 36.31 (67) at 9 institutions Nene Goose - 37.35.4 (76) at 20 institutions Crested Screamer - 55.44.10 (109) at 49 institutions Coscoroba Swan – 18.17 (35) at 18 institutions Marbled Teal - 94.94.00 (188) at 40 institutions West Indian Whistling Duck - 38.26.2 (66) at 19 institutions Madagascar Teal 40.48.6 (94) at 15 institutions Orinoco Goose - 47.35.2 (84) at 21 institutions Red SSP Red-breasted Goose - 32.28.7 (67) at 17 institutions Spotted Whistling Duck - 18.9 (27) at 6 institutions Indian Pygmy Goose - 13.12.3 (28) at 11 institutions Candidate Program Species Baer’s Pochard - 29.25.1 (55) at 8 institutions -

Parasitic Helminths and Arthropods of Fulvous Whistling-Ducks (Dendrocygna Bicolor) in Southern Florida
J. Helminthol. Soc. Wash. 61(1), 1994, pp. 84-88 Parasitic Helminths and Arthropods of Fulvous Whistling-Ducks (Dendrocygna bicolor) in Southern Florida DONALD J. FORRESTER,' JOHN M. KINSELLA,' JAMES W. MERTiNS,2 ROGER D. PRICE,3 AND RICHARD E. TuRNBULL4 5 1 Department of Infectious Diseases, College of Veterinary Medicine, University of Florida, Gainesville, Florida 32610, 2 U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services, National Veterinary Services Laboratories, P.O. Box 844, Ames, Iowa 50010, 1 Department of Entomology, University of Minnesota, St. Paul, Minnesota 55108, and 4 Florida Game and Fresh Water Fish Commission, Okeechobee, Florida 34974 ABSTRACT: Thirty fulvous whistling-ducks (Dendrocygna bicolor) collected during 1984-1985 from the Ever- glades Agricultural Area of southern Florida were examined for parasites. Twenty-eight species were identified and included 8 trematodes, 6 cestodes, 1 nematode, 4 chewing lice, and 9 mites. All parasites except the 4 species of lice and 1 of the mites are new host records for fulvous whistling-ducks. None of the ducks were infected with blood parasites. Every duck was infected with at least 2 species of helminths (mean 4.2; range 2- 8 species). The most common helminths were the trematodes Echinostoma trivolvis and Typhlocoelum cucu- merinum and 2 undescribed cestodes of the genus Diorchis, which occurred in prevalences of 67, 63, 50, and 50%, respectively. Only 1 duck was free of parasitic arthropods; each of the other 29 ducks was infested with at least 3 species of arthropods (mean 5.3; range 3-9 species). The most common arthropods included an undescribed feather mite (Ingrassia sp.) and the chewing louse Holomenopon leucoxanthum, both of which occurred in 97% of the ducks. -

A Preliminary Risk Assessment of Cane Toads in Kakadu National Park Scientist Report 164, Supervising Scientist, Darwin NT
supervising scientist 164 report A preliminary risk assessment of cane toads in Kakadu National Park RA van Dam, DJ Walden & GW Begg supervising scientist national centre for tropical wetland research This report has been prepared by staff of the Environmental Research Institute of the Supervising Scientist (eriss) as part of our commitment to the National Centre for Tropical Wetland Research Rick A van Dam Environmental Research Institute of the Supervising Scientist, Locked Bag 2, Jabiru NT 0886, Australia (Present address: Sinclair Knight Merz, 100 Christie St, St Leonards NSW 2065, Australia) David J Walden Environmental Research Institute of the Supervising Scientist, GPO Box 461, Darwin NT 0801, Australia George W Begg Environmental Research Institute of the Supervising Scientist, GPO Box 461, Darwin NT 0801, Australia This report should be cited as follows: van Dam RA, Walden DJ & Begg GW 2002 A preliminary risk assessment of cane toads in Kakadu National Park Scientist Report 164, Supervising Scientist, Darwin NT The Supervising Scientist is part of Environment Australia, the environmental program of the Commonwealth Department of Environment and Heritage © Commonwealth of Australia 2002 Supervising Scientist Environment Australia GPO Box 461, Darwin NT 0801 Australia ISSN 1325-1554 ISBN 0 642 24370 0 This work is copyright Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Supervising Scientist Requests and inquiries concerning reproduction -

Waterfowl of North America: WHISTLING DUCKS Tribe Dendrocygnini
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Waterfowl of North America, Revised Edition (2010) Papers in the Biological Sciences 2010 Waterfowl of North America: WHISTLING DUCKS Tribe Dendrocygnini Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciwaterfowlna Part of the Ornithology Commons Johnsgard, Paul A., "Waterfowl of North America: WHISTLING DUCKS Tribe Dendrocygnini" (2010). Waterfowl of North America, Revised Edition (2010). 8. https://digitalcommons.unl.edu/biosciwaterfowlna/8 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Waterfowl of North America, Revised Edition (2010) by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. WHISTLING DUCKS Tribe Dendrocygnini Whistling ducks comprise a group of nine species that are primarily of tropical and subtropical distribution. In common with the swans and true geese (which with them comprise the subfamily Anserinae), the included spe cies have a reticulated tarsal surface pattern, lack sexual dimorphism in plum age, produce vocalizations that are similar or identical in both sexes, form relatively permanent pair bonds, and lack complex pair-forming behavior pat terns. Unlike the geese and swans, whistling ducks have clear, often melodious whistling voices that are the basis for their group name. The alternative name, tree ducks, is far less appropriate, since few of the species regularly perch or nest in trees. All the species have relatively long legs and large feet that extend beyond the fairly short tail when the birds are in flight. -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
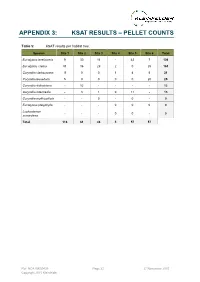
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential