Innovative Immunization Strategies for Antivenom Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Eight Years Later, Mario Guerra Says Goodbye
Local Sean Ashton Singer, conductor obituaries takes oath Meg Zeleny See Page 3 See Page 2 See Page 8 Thursday, Dec. 11, 2014 Vol. 13 No. 35 8301 E. Florence Ave., Suite 100, Downey, CA 90240 SHARED STORIES: THE TIES THAT BIND Eight years later, Lost in Los Angeles Weekend Mario Guerra says goodbye at a Noemi Rabina looks back with humor on what became an exhausting ordeal Glance as she and her husband tried to drive home after paying their property tax in • Two-term councilman ⁰ person. Shared Stories is a weekly column featuring articles by participants in 64 reflects on eight years as a city Friday a writing class at the Norwalk Senior Center. Bonnie Mansell is the instructor representative. for this free class offered through the Cerritos College Adult Education Program. Curated by Carol Kearns 67⁰ By Noemi Rabina Saturday By Mario Guerra December 10 is the deadline for paying property tax. But where is the bill? We don’t remember if we have received one. If we are late in our payment, we will have to pay the penalty. My husband decided to go to Los Angeles to pay ⁰ our bill in person. Dear Downey Family and Sunday 66 He tried to figure out how to take the bus because neither of us drive on the Friends, freeway. I won’t let him go alone to Los Angeles and get lost, so I studied the The last eight years have been map and figured out how I could drive on surface streets. Even at turtle speed, it truly memorable and filled with would be better to get there rather than stay at home and worry about him. -

Convergent Recruitment of Knottin and Defensin Peptide Scaffolds Into the Venom of Predatory Assassin Flies
Journal Pre-proof Weaponisation ‘on the fly’: convergent recruitment of knottin and defensin peptide scaffolds into the venom of predatory assassin flies Jiayi Jin, Akello J. Agwa, Tibor G. Szanto, Agota Csóti, Gyorgy Panyi, Christina I. Schroeder, Andrew A. Walker, Glenn F. King PII: S0965-1748(19)30425-4 DOI: https://doi.org/10.1016/j.ibmb.2019.103310 Reference: IB 103310 To appear in: Insect Biochemistry and Molecular Biology Received Date: 8 October 2019 Revised Date: 12 December 2019 Accepted Date: 16 December 2019 Please cite this article as: Jin, J., Agwa, A.J., Szanto, T.G., Csóti, A., Panyi, G., Schroeder, C.I, Walker, A.A., King, G.F., Weaponisation ‘on the fly’: convergent recruitment of knottin and defensin peptide scaffolds into the venom of predatory assassin flies Insect Biochemistry and Molecular Biology, https:// doi.org/10.1016/j.ibmb.2019.103310. This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. © 2019 Published by Elsevier Ltd. Intended for submission to Insect Biochemistry and Molecular Biology Special Issue on Active Peptides in Insects Weaponisation ‘on the fly’: convergent recruitment of knottin and defensin peptide scaffolds into the venom of predatory assassin flies Jiayi Jin 1, Akello J. -
Snake Bite Protocol
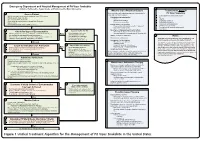
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Charybdotoxin and Noxiustoxin, Two Homologous Peptide Inhibitors of the K+(Ca2+) Channel
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Volume 226, number 2, 280-284 FEB 05447 January 1988 Charybdotoxin and noxiustoxin, two homologous peptide inhibitors of the K+(Ca2+) channel Hector H. Valdivia*, Jeffrey S. Smith*, Brian M. Martin+, Roberto Coronado* and Lourival D. Possani*’ *Department of Physiology and Molecular Biophysics, Baylor College of Medicine, I Baylor Plaza, Houston, TX 77030, +National Institute of Mental Health, Molecular Neurogenetics Unit, Clinical Neuroscience Branch, Building IO 3016. NIH, Bethesda, MD 20892, USA and “Departamento de Bioquimica, Centro de Investigation sobre Ingenieria Genetica y Biotecnologia. Universidad National Autonoma de Mexico. Apartado Postal 510-3 Cuernavaca, Morelos 62271, Mexico Received 30 October 1987 We show that noxiustoxin (NTX), like charybdotoxin (CTX) described by others, affects CaZt-activated K+ channels of skeletal muscle (K+(Ca2+) channels). Chemical characterization of CTX shows that it is similar to NTX. Although the amino-terminal amino acid of CTX is not readily available, the molecule was partially sequenced after CNBr cleavage. A decapeptide corresponding to the C-terminal region of NTX shows 60% homology to that of CTX, maintaining the cysteine residues at the same positions. While CTX blocks the K+(Ca2+) channels with a & of 1-3 nM, for NTX it is approx. 450 nM. Both peptides can interact simultaneously with the same channel. NTX and CTX promise to be good tools for channel isolation. -

K Channels As Targets for Specific Immunomodulation
Review TRENDS in Pharmacological Sciences Vol.25 No.5 May 2004 K1 channels as targets for specific immunomodulation K. George Chandy1, Heike Wulff2, Christine Beeton1, Michael Pennington3, George A. Gutman1 and Michael D. Cahalan1 1Department of Physiology and Biophysics, University of California, Irvine, CA 92697, USA 2Department of Pharmacology and Toxicology, University of California, Davis, CA 95616, USA 3Bachem Bioscience, King of Prussia, PA 19406, USA 21 The voltage-gated Kv1.3 channel and the Ca -activated gene encodes the lymphocyte KV channel [8,9].An IKCa1 K1 channel are expressed in T cells in a distinct intermediate-conductance Ca2þ-activated Kþ channel pattern that depends on the state of lymphocyte acti- was identified in T cells in 1992 [10–12], and shown to vation and differentiation. The channel phenotype be a product of the KCNN4 (IKCa1, KCa3.1; http://www. changes during the progression from the resting to the iuphar-db.org/iuphar-ic/KCa.html) gene in 1997 [13]. activated cell state and from naı¨ve to effector memory Subsequent studies by our group identified calmodulin cells, affording promise for specific immunomodulatory as the Ca2þ sensor of the IKCa1 channel [14]. The salient actions of K1 channel blockers. In this article, we review features of both channels were summarized in a recent the functional roles of these channels in both naı¨ve cells review [15]. and memory cells, describe the development of selec- Following the discovery that Kþ channels are essential tive inhibitors of Kv1.3 and IKCa1 channels, and provide for T-cell function, several other Kþ channels have been a rationale for the potential therapeutic use of these implicated in the proliferation of a wide variety of normal inhibitors in immunological disorders. -

Production of Scorpion Antivenom From
Received: March 6, 2007 J. Venom. Anim. Toxins incl. Trop. Dis. Accepted: May 9, 2007 V.13, n.4, p.844-856, 2007. Abstract published online: May 9, 2007 Original paper. Full paper published online: November 30, 2007 ISSN 1678-9199. COMPARISON OF PROTEINS, LETHALITY AND IMMUNOGENIC COMPOUNDS OF Androctonus crassicauda (OLIVIER, 1807) (SCORPIONES: BUTHIDAE) VENOM OBTAINED BY DIFFERENT METHODS OZKAN O. (1, 2), KAR S. (2), GÜVEN E. (2) ERGUN G. (3) (1) Refik Saydam Hygiene Center, Ankara, Turkey; (2) Department of Entomology, Faculty of Veterinary Medicine, Ankara, Turkey; (3) Department of Statistics, Faculty of Sciences, Hacettepe University, Ankara, Turkey. ABSTRACT: Scorpions are venomous arthropods of the class Arachnida and are considered relatives of spiders, ticks and mites. There are approximately 1,500 species of scorpions worldwide, which are characterized by an elongated body and a segmented tail that ends in a venomous stinger. No specific treatment is available for scorpion envenomation, except for the use of antivenom. The current study aimed at comparing protein content and lethality of Androctonus crassicauda venom extracted by two different methods (electric stimulation and maceration of telsons). The LD50 calculated by probit analysis was 1.1mg/kg for venom obtained by electric stimulation and 39.19mg/kg for venom obtained by maceration of telsons. In the electrophoretic analysis, protein bands of the venom sample obtained by electric stimulation were between 12 and 53kDa (total: five bands), and those of venom extracted by maceration appeared as multiple protein bands, relative to the other venom sample. Low-molecular-weight proteins, revealed by western blotting, played an important immunogenic role in the production of antivenom. -

Recent Advances in Research on Widow Spider Venoms and Toxins
Review Recent Advances in Research on Widow Spider Venoms and Toxins Shuai Yan and Xianchun Wang * Received: 2 August 2015; Accepted: 16 November 2015; Published: 27 November 2015 Academic Editors: Richard J. Lewis and Glenn F. King Key Laboratory of Protein Chemistry and Developmental Biology of Ministry of Education, College of Life Sciences, Hunan Normal University, Changsha 410081, China; [email protected] * Correspondence: [email protected]; Tel.: +86-731-8887-2556 Abstract: Widow spiders have received much attention due to the frequently reported human and animal injures caused by them. Elucidation of the molecular composition and action mechanism of the venoms and toxins has vast implications in the treatment of latrodectism and in the neurobiology and pharmaceutical research. In recent years, the studies of the widow spider venoms and the venom toxins, particularly the α-latrotoxin, have achieved many new advances; however, the mechanism of action of the venom toxins has not been completely clear. The widow spider is different from many other venomous animals in that it has toxic components not only in the venom glands but also in other parts of the adult spider body, newborn spiderlings, and even the eggs. More recently, the molecular basis for the toxicity outside the venom glands has been systematically investigated, with four proteinaceous toxic components being purified and preliminarily characterized, which has expanded our understanding of the widow spider toxins. This review presents a glance at the recent advances in the study on the venoms and toxins from the Latrodectus species. Keywords: widow spider; venom; toxin; latrotoxin; latroeggtoxin; advance 1. Introduction Latrodectus spp. -

Mechanistic Insights Into Functional Characteristics of Native Crotamine
Toxicon 146 (2018) 1e12 Contents lists available at ScienceDirect Toxicon journal homepage: www.elsevier.com/locate/toxicon Mechanistic insights into functional characteristics of native crotamine Daniel Batista da Cunha a, Ana Vitoria Pupo Silvestrini a, Ana Carolina Gomes da Silva a, Deborah Maria de Paula Estevam b,Flavia Lino Pollettini b, Juliana de Oliveira Navarro a, Armindo Antonio^ Alves a, Ana Laura Remedio Zeni Beretta a, * Joyce M. Annichino Bizzacchi c, Lilian Cristina Pereira d, Maurício Ventura Mazzi a, a Graduate Program in Biomedical Sciences Hermínio Ometto University Center, UNIARARAS, 7 Av. Dr. Maximiliano Baruto, 500, CEP 13607-339, Araras, SP, Brazil b Graduate Program in Agrarian and Veterinary Sciences, State University Paulista Júlio de Mesquita Filho-UNESP, Jaboticabal, SP, Brazil c Blood Hemostasis Laboratory, Faculty of Medical Sciences, State University of Campinas, Campinas, SP, Brazil d Department of Bioprocesses and Biotechnology, Faculty of Agronomic Sciences, State University Paulista Júlio Mesquita Filho-UNESP, Botucatu, SP, Brazil article info abstract Article history: The chemical composition of snake venoms is a complex mixture of proteins and peptides that can be Received 4 October 2017 pharmacologically active. Crotamine, a cell-penetrating peptide, has been described to have antimicro- Received in revised form bial properties and it exerts its effects by interacting selectively with different structures, inducing 6 March 2018 changes in the ion flow pattern and cellular responses. However, its real therapeutic potential is not yet Accepted 20 March 2018 fully known. Bearing in mind that crotamine is a promising molecule in therapeutics, this study inves- Available online 21 March 2018 tigated the action of purified molecule in three aspects: I) antibacterial action on different species of clinical interest, II) the effect of two different concentrations of the molecule on platelet aggregation, and Keywords: fi Crotalus durissus terrificus III) its effects on isolated mitochondria. -

Bioinformatic Characterizations and Prediction of K+ and Na+ Ion Channels Effector Toxins
Bioinformatic characterizations and prediction of K+ and Na+ ion channels effector toxins. Rima Soli, Belhassen Kaabi, Mourad Barhoumi, Mohamed El-Ayeb, Najet Srairi-Abid To cite this version: Rima Soli, Belhassen Kaabi, Mourad Barhoumi, Mohamed El-Ayeb, Najet Srairi-Abid. Bioinformatic characterizations and prediction of K+ and Na+ ion channels effector toxins.. BMC Pharmacology, BioMed Central, 2009, 9, pp.4. 10.1186/1471-2210-9-4. pasteur-00612552 HAL Id: pasteur-00612552 https://hal-riip.archives-ouvertes.fr/pasteur-00612552 Submitted on 2 Aug 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. BMC Pharmacology BioMed Central Research article Open Access Bioinformatic characterizations and prediction of K+ and Na+ ion channels effector toxins Rima Soli†1, Belhassen Kaabi*†1,3, Mourad Barhoumi1, Mohamed El-Ayeb2 and Najet Srairi-Abid2 Address: 1Laboratory of Epidemiology and Ecology of Parasites, Institut Pasteur de Tunis, Tunis, Tunisia, 2Laboratory of Venom and Toxins, Institut Pasteur de Tunis, Tunis, Tunisia and 3Research and Teaching Building, -

Spider Woman
a reporter at lARgE spider woman Hunting venomous species in the basements of Los Angeles. bY buRKHARd BilgER arly one morning last year, when the of the brown recluse, but larger and lady! Spider lady! Come to the front!” streets of downtown Los Angeles more venomous. Sometime in the late Torres was standing by the cash register, wereE still mostly deserted, a strange figure nineteen-sixties, apparently, their ances- her hands on her hips. She made Binford appeared in the Goodwill store at 235 tors had ridden to California in costume scrawl out a waiver on a legal pad, then led South Broadway, next door to the Gua- crates owned by a troupe of Shakespear- her down a long, dingy hallway to the dalupe Wedding Chapel. She had on ten- ean actors from Brazil. A year or two basement door. “It’s your own risk,” she nis shoes, dungarees, and a faded blue later, they were discovered at a theatre said, pointing down the stairwell. “If I T-shirt, and was outfitted as if for a safari in the L.A. suburb of Sierra Madre don’t hear from you in two days, I call the or a spelunking expedition. A khaki vest and promptly triggered a citywide panic. authorities.” was stuffed with empty plastic vials; a “50 DeadlY SpideRS FOUND,” a front- black duffelbag across her shoulders held page headline in the Los Angeles Times piders have a bad reputation, largely a pair of high-tech headlamps, a digital announced on June 7, 1969. “VENom undeserved. The great majority aren’t camera, and a venom extractor. -

Α-Neurexins Together Withα2δ-1 Auxiliary Subunits Regulate Ca
The Journal of Neuroscience, September 19, 2018 • 38(38):8277–8294 • 8277 Cellular/Molecular ␣-Neurexins Together with ␣2␦-1 Auxiliary Subunits 2ϩ Regulate Ca Influx through Cav2.1 Channels X Johannes Brockhaus,1* Miriam Schreitmu¨ller,1* Daniele Repetto,1 Oliver Klatt,1,2 XCarsten Reissner,1 X Keith Elmslie,3 Martin Heine,2 and XMarkus Missler1,4 1Institute of Anatomy and Molecular Neurobiology, Westfa¨lische Wilhelms-University, 48149 Mu¨nster, Germany, 2Molecular Physiology Group, Leibniz- Institute of Neurobiology, 39118 Magdeburg, Germany, 3Department of Pharmacology, AT Still University of Health Sciences, Kirksville, Missouri 63501, and 4Cluster of Excellence EXC 1003, Cells in Motion, 48149 Mu¨nster, Germany Action potential-evoked neurotransmitter release is impaired in knock-out neurons lacking synaptic cell-adhesion molecules ␣-neurexins (␣Nrxns), the extracellularly longer variants of the three vertebrate Nrxn genes. Ca 2ϩ influx through presynaptic high- ␣ ␦ voltage gated calcium channels like the ubiquitous P/Q-type (CaV2.1) triggers release of fusion-ready vesicles at many boutons. 2 Auxiliary subunits regulate trafficking and kinetic properties of CaV2.1 pore-forming subunits but it has remained unclear if this involves ␣Nrxns. Using live cell imaging with Ca 2ϩ indicators, we report here that the total presynaptic Ca 2ϩ influx in primary hippocampal ␣ neurons of Nrxn triple knock-out mice of both sexes is reduced and involved lower CaV2.1-mediated transients. This defect is accom- ␣ ␦ panied by lower vesicle release, reduced synaptic abundance of CaV2.1 pore-forming subunits, and elevated surface mobility of 2 -1 on axons. Overexpression of Nrxn1␣ in ␣Nrxn triple knock-out neurons is sufficient to restore normal presynaptic Ca 2ϩ influx and synaptic vesicle release. -

Venom Week 2012 4Th International Scientific Symposium on All Things Venomous
17th World Congress of the International Society on Toxinology Animal, Plant and Microbial Toxins & Venom Week 2012 4th International Scientific Symposium on All Things Venomous Honolulu, Hawaii, USA, July 8 – 13, 2012 1 Table of Contents Section Page Introduction 01 Scientific Organizing Committee 02 Local Organizing Committee / Sponsors / Co-Chairs 02 Welcome Messages 04 Governor’s Proclamation 08 Meeting Program 10 Sunday 13 Monday 15 Tuesday 20 Wednesday 26 Thursday 30 Friday 36 Poster Session I 41 Poster Session II 47 Supplemental program material 54 Additional Abstracts (#298 – #344) 61 International Society on Thrombosis & Haemostasis 99 2 Introduction Welcome to the 17th World Congress of the International Society on Toxinology (IST), held jointly with Venom Week 2012, 4th International Scientific Symposium on All Things Venomous, in Honolulu, Hawaii, USA, July 8 – 13, 2012. This is a supplement to the special issue of Toxicon. It contains the abstracts that were submitted too late for inclusion there, as well as a complete program agenda of the meeting, as well as other materials. At the time of this printing, we had 344 scientific abstracts scheduled for presentation and over 300 attendees from all over the planet. The World Congress of IST is held every three years, most recently in Recife, Brazil in March 2009. The IST World Congress is the primary international meeting bringing together scientists and physicians from around the world to discuss the most recent advances in the structure and function of natural toxins occurring in venomous animals, plants, or microorganisms, in medical, public health, and policy approaches to prevent or treat envenomations, and in the development of new toxin-derived drugs.