Cervical Cancer 2007 Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidance for the Format and Content of the Protocol of Non-Interventional
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

Tnm Frequently Asked Questions (Faq’S)
TNM FREQUENTLY ASKED QUESTIONS (FAQ’S) The TNM Project Committee receives questions concerning the use of TNM and how to interpret rules in specific situations. Some questions and answers are listed below by category for your convenience. These FAQs can th also be found in the TNM Supplement: a Commentary on Uniform Use, 4 Edition, 2012 (edited by Ch. Wittekind, C. Compton, J. Brierley, L. H. Sobin). Advice on further questions may be obtained from the TNM Helpdesk by accessing the TNM Classification of Malignant Tumours page at the UICC website www.uicc.org TABLE OF CONTENTS GENERAL QUESTIONS AJCC versus UICC TNM ................................................................................................................3 In situ carcinoma .............................................................................................................................3 Pathological Versus Clinical TNM ...................................................................................................3 When in Doubt ................................................................................................................................4 R Classification ...............................................................................................................................4 R Classification and Tis ..................................................................................................................5 Positive Cytology ............................................................................................................................5 -

Ministry of Education and Science of Ukraine Sumy State University 0
Ministry of Education and Science of Ukraine Sumy State University 0 Ministry of Education and Science of Ukraine Sumy State University SPLANCHNOLOGY, CARDIOVASCULAR AND IMMUNE SYSTEMS STUDY GUIDE Recommended by the Academic Council of Sumy State University Sumy Sumy State University 2016 1 УДК 611.1/.6+612.1+612.017.1](072) ББК 28.863.5я73 С72 Composite authors: V. I. Bumeister, Doctor of Biological Sciences, Professor; L. G. Sulim, Senior Lecturer; O. O. Prykhodko, Candidate of Medical Sciences, Assistant; O. S. Yarmolenko, Candidate of Medical Sciences, Assistant Reviewers: I. L. Kolisnyk – Associate Professor Ph. D., Kharkiv National Medical University; M. V. Pogorelov – Doctor of Medical Sciences, Sumy State University Recommended for publication by Academic Council of Sumy State University as а study guide (minutes № 5 of 10.11.2016) Splanchnology Cardiovascular and Immune Systems : study guide / С72 V. I. Bumeister, L. G. Sulim, O. O. Prykhodko, O. S. Yarmolenko. – Sumy : Sumy State University, 2016. – 253 p. This manual is intended for the students of medical higher educational institutions of IV accreditation level who study Human Anatomy in the English language. Посібник рекомендований для студентів вищих медичних навчальних закладів IV рівня акредитації, які вивчають анатомію людини англійською мовою. УДК 611.1/.6+612.1+612.017.1](072) ББК 28.863.5я73 © Bumeister V. I., Sulim L G., Prykhodko О. O., Yarmolenko O. S., 2016 © Sumy State University, 2016 2 Hippocratic Oath «Ὄμνυμι Ἀπόλλωνα ἰητρὸν, καὶ Ἀσκληπιὸν, καὶ Ὑγείαν, καὶ Πανάκειαν, καὶ θεοὺς πάντας τε καὶ πάσας, ἵστορας ποιεύμενος, ἐπιτελέα ποιήσειν κατὰ δύναμιν καὶ κρίσιν ἐμὴν ὅρκον τόνδε καὶ ξυγγραφὴν τήνδε. -

Incidence, Morbidity and Mortality of Patients with Achalasia in England: Findings from a Nationwide Hospital Database and 4 Million Population Based Data
Incidence, morbidity and mortality of patients with achalasia in England: findings from a nationwide hospital database and 4 million population based data Appendix A: International Classification of Disease codes for Achalasia (HES) K22 – Achalasia of cardia Appendix B: Read codes (The Health Improvement Network) Appendix B1: Achalasia codes Clinical code Description J100.00 Achalasia of cardia Appendix B2: Clinical codes used to identify Hypertension, Diabetes and lipid lowering drugs Diabetes Clinical code Description C10..00 Diabetes mellitus C100.00 Diabetes mellitus with no mention of complication C100000 Diabetes mellitus, juvenile type, no mention of complication C100011 Insulin dependent diabetes mellitus C100100 Diabetes mellitus, adult onset, no mention of complication C100111 Maturity onset diabetes C100112 Non-insulin dependent diabetes mellitus C100z00 Diabetes mellitus NOS with no mention of complication C101.00 Diabetes mellitus with ketoacidosis C101000 Diabetes mellitus, juvenile type, with ketoacidosis C101100 Diabetes mellitus, adult onset, with ketoacidosis C101y00 Other specified diabetes mellitus with ketoacidosis C101z00 Diabetes mellitus NOS with ketoacidosis C102.00 Diabetes mellitus with hyperosmolar coma C102000 Diabetes mellitus, juvenile type, with hyperosmolar coma C102100 Diabetes mellitus, adult onset, with hyperosmolar coma C102z00 Diabetes mellitus NOS with hyperosmolar coma C103.00 Diabetes mellitus with ketoacidotic coma C103000 Diabetes mellitus, juvenile type, with ketoacidotic coma C103100 Diabetes -

Lymphadenectomy in Epithelial Ovarian Cancer: Clinico-Pathological Predictive Factors for Nodal Involvement
LYMPHADENECTOMY IN EPITHELIAL OVARIAN CANCER: CLINICO-PATHOLOGICAL PREDICTIVE FACTORS FOR NODAL INVOLVEMENT Thesis Submitted for partial fulfillment of MD Degree In Surgical Oncology Presented by Tamer Mostafa Manie M.B.B.Ch,M.Sc. Under supervision of Prof. Dr.Sherif Fouad Naguib Prof. of surgical oncology National Cancer Institute – Cairo University Dr. Iman Gouda Farahat Assistant Prof. of Pathology National Cancer Institute – Cairo University Dr. Abdel Hamid Hussein Ezzat Assistant Prof. of surgical oncology National Cancer Institute – Cairo University Dr. Tarek Sherif El Baradie Lecturer of surgical oncology National Cancer Institute – Cairo University Cairo University 2014 Table of contents Page INTRODUCTION 1 AIM OF WORK 5 REVIEW OF LITERATURE 6 Anatomy 6 Mechanism of tumor dissemination via the lymphatic system 16 Pathology 18 Diagnosis 27 Impact on diagnosis 49 Para-aortic dissection 55 Pelvic lymphadenectomy 65 Laparoscopic lymphadenectomy 68 Robotic surgery in lymphadenectomy 71 Complications of lymphadenectomy 78 PATIENTS AND METHODS 84 RESULTS 94 DISCUSSION 104 SUMMARY 136 CONCLUSION 139 REFERENCES 142 ARABIC SUMMARY 176 Introduction The International Federation of Obstetrics and Gynecology (FIGO) had indicated that pelvic and para-aortic lymph node sampling is an integral part of the staging system of ovarian cancer. The FIGO staging classification was amended to include a sub-stage for node involvement to reflect the prognostic significance of metastatic spread to pelvic and para-aortic lymph nodes [1] . Lymphadenectomy is an integral part in the management of ovarian cancer, and it has a potential role in both staging and retroperitoneal debulking [2]. In disease confined to one or both ovaries, positive nodes resulted in upstaging from stage I to stage IIIC [3] . -

18.1 Acute Postoperative Complications M
18 Postoperative Complications 18.1 Acute Postoperative Complications M. Seitz, B. Schlenker, Ch. Stief 18.1.1 Postoperative Bleeding 364 18.1.5 Abdominal Wound Dehiscence 403 18.1.1.1 Overview 364 18.1.5.1 Synonyms 403 18.1.1.2 Incidence and Risk Factors 365 18.1.5.2 Overview and Incidence 403 18.1.1.3 Detection and Clinical Signs 365 18.1.5.3 Risk Factors 404 18.1.1.4 Workup 366 18.1.5.4 Clinical Signs and Complications 404 18.1.1.5 Management 366 18.1.5.5 Prevention 405 18.1.1.6 Special Conditions 371 18.1.5.6 Management 405 18.1.2 Chest Pain and Dyspnea 373 18.1.6 Chylous Ascites 410 18.1.2.1 Overview 373 18.1.6.1 Overview 410 18.1.2.2 Cardiovascular System Disorders 373 18.1.6.2 Risk Factors and Pathogenesis 410 18.1.2.3 Postoperative Pulmonary Complications 373 18.1.6.3 Prevention 412 Pulmonary Embolism 373 18.1.6.4 Detection and Workup 413 Pleural Effusions 375 18.1.6.5 Management 413 Atelectasis 376 Infection/Pneumonia 376 18.1.7 Deep Venous Thrombosis 414 Tube Thoracostomy 376 18.1.7.1 Overview and Incidence 414 18.1.3 Acute Abdomen 377 18.1.7.2 Risk Factors 414 18.1.3.1 Initial Management 377 18.1.7.3 Detection and Clinical Findings 415 18.1.4 18.1.7.4 Management 415 Postoperative Fever 378 Unfractionated Heparin 415 18.1.4.1 Overview 378 Low-Molecular-Weight Heparin 415 18.1.4.2 Incidence 379 Long-Term Therapy 416 18.1.4.3 Definition 379 18.1.4.4 Risk Factors and Prevention 379 18.1.8 Lymphoceles 416 18.1.4.5 Detection and Work-Up 382 18.1.8.1 Anatomy and Physiology 416 Pulmonary 382 18.1.8.2 Overview 419 Urinary Tract 382 -

Anatomy and Physiology Model Guide Book
Anatomy & Physiology Model Guide Book Last Updated: August 8, 2013 ii Table of Contents Tissues ........................................................................................................................................................... 7 The Bone (Somso QS 61) ........................................................................................................................... 7 Section of Skin (Somso KS 3 & KS4) .......................................................................................................... 8 Model of the Lymphatic System in the Human Body ............................................................................. 11 Bone Structure ........................................................................................................................................ 12 Skeletal System ........................................................................................................................................... 13 The Skull .................................................................................................................................................. 13 Artificial Exploded Human Skull (Somso QS 9)........................................................................................ 14 Skull ......................................................................................................................................................... 15 Auditory Ossicles .................................................................................................................................... -

Scintigraphy of Lymph Nodes K. Zum Winkel, H
107 Lymphology 10 (1977) 107-114 ©Georg Thieme Verlag Stuttgart Scintigraphy of Lymph Nodes K. zum Winkel, H.-J. Hermann Klinikum der Universltat Heidelberg, Zentrum Radiologie, Abt. Allgem. Radiologie mit Poliklinik {Arztl. Direktor: Prof. Dr. K. zum Winkel) Summary culation via the thoracic duct, and are depo The basic considerations, pharmacology, techniques, sited in the reticuloendothelial system of the and clinical results of lymphoscintigraphy performed liver. after the interstitial application of radiocolloids or after i.v. injection of radiogallium or radiobleomycin Colloidal 198 aurum is a suitable radiopharma are described. The advantages and disadvantages are ceutical because of its small particle size (aver summarized, and the indications are given. age: 5 J.Lffi) as well as its stable and reliable preparation. However, the physical half-life Scintigraphy of the lymphatics after radiophar of 2. 7 days is long and the radiation dose for maceuticals have been subcutaneously applied the patient is rather high due to beta radia provide information about the circulation with tion. Substances labeled with 99m technetium in the lymphatic vessels and the phagocytic are important (I 0) because of the short half function of the lymph nodes. On the other life of 6 hours and the lack of beta radiation: hand, the metabolism of lymphatic tumors Today kits containing sulphur microcolloids can be studied after intravenously! injected are available which can be labeled with 99mTc 67 gallium citrate or radioactive labeled bleo pertechnetate within a few minutes. The aver mycin. age particle size is 3 J.Lin. In spite of these ad By means of scintigraphy, the following topo vantages, the labeling of 99mTc is less stable graphic groups of lymph vessels and nodes than that. -

Read Code Description 14L.. H/O: Drug Allergy 158.. H/O: Abnormal Uterine Bleeding 16C2
Read Code Description 14L.. H/O: drug allergy 158.. H/O: abnormal uterine bleeding 16C2. Backache 191.. Tooth symptoms 191Z. Tooth symptom NOS 1927. Dry mouth 198.. Nausea 199.. Vomiting 19C.. Constipation 1A23. Incontinence of urine 1A32. Cannot pass urine - retention 1B1G. Headache 1B62. Syncope/vasovagal faint 1B75. Loss of vision 1BA2. Generalised headache 1BA3. Unilateral headache 1BA4. Bilateral headache 1BA5. Frontal headache 1BA6. Occipital headache 1BA7. Parietal headache 1BA8. Temporal headache 1C13. Deafness 1C131 Unilateral deafness 1C132 Partial deafness 1C133 Bilateral deafness 1C14. "Blocked ear" 1C15. Popping sensation in ear 1C1Z. Hearing symptom NOS 22J.. O/E - dead 22J4. O/E - dead - sudden death 22L4. O/E - Wound infected 2542. O/E - dental caries 2554. O/E - gums - blue line 2555. O/E - hypertrophy of gums 2FF.. O/E - skin ulcer 2I14. O/E - a rash 39C0. Pressure sore 39C1. Superficial pressure sore 39C2. Deep pressure sore 62... Patient pregnant 6332. Single stillbirth 66G4. Allergy drug side effect 72001 Enucleation of eyeball 7443. Exteriorisation of trachea 744D. Tracheo-oesophageal puncture 7511. Surgical removal of tooth 75141 Root canal therapy to tooth 7610. Total excision of stomach 7645. Creation of ileostomy 773C. Other operations on bowel 773Cz Other operation on bowel NOS 7826. Incision of bile duct 7840. Total excision of spleen 7B01. Total nephrectomy 7C032 Unilateral total orchidectomy - unspecified 7E117 Left salpingoophorectomy 7E118 Right salpingectomy 7E119 Left salpingectomy 7G321 Avulsion of nail 7H220 Exploratory laparotomy 7J174 Manipulation of mandible 8HG.. Died in hospital 94B.. Cause of death A.... Infectious and parasitic diseases A0... Intestinal infectious diseases A00.. Cholera A000. -

Chapter 2 Initial Treatment
Page-17 Chapter 2■ Initial Treatment Overview In 1988, the International Federation of Gynecology and Obstetrics (FIGO) adopted a new surgical staging classification system for uterine body cancer, necessitating the selection of surgical procedures that included retroperitoneal lymph node examination for stage determination. Uterine body cancer is known to have lower radiosensitivity than cervical cancer. In addition, the establishment of a standard treatment using anticancer drugs has taken longer for uterine body cancer as compared to ovarian cancer. Therefore, the first choice treatment for uterine body cancer is surgery. Radiotherapy is suitable for inoperable patients, including patients with serious medical conditions and the elderly. Hysterectomy In 2005, the Japan Gynecologic Oncology Group (JGOG) conducted a questionnaire survey1 on uterine body cancer. For the standard surgical procedure for uterine body cancer, one third of institutions replied that they use total hysterectomy, another third replied that they use radical hysterectomy, and the remaining third replied that they modify the procedure according to the stage as estimated preoperatively. Approximately 70% of institutions answered that they do not perform radical hysterectomy for uterine body cancer. The differences in surgical techniques themselves among institutions are one of the reasons for difficulty in obtaining a consensus on surgical techniques. We can presume that radical hysterectomy is performed using a similar technique throughout Japan. However, differences between institutions are thought to exist in procedures such as extended total hysterectomy and modified radical hysterectomy. Similarly, an Italian report indicated that there were large differences in surgical techniques between institutions.2 It is also necessary to consider the differences in techniques between Japan and Western countries. -

Posterior Mediastinum: Mediastinal Organs 275
104750_S_265_290_Kap_4:_ 05.01.2010 10:43 Uhr Seite 275 Posterior Mediastinum: Mediastinal Organs 275 1 Internal jugular vein 2 Right vagus nerve 3 Thyroid gland 4 Right recurrent laryngeal nerve 5 Brachiocephalic trunk 6 Trachea 7 Bifurcation of trachea 8 Right phrenic nerve 9 Inferior vena cava 10 Diaphragm 11 Left subclavian artery 12 Left common carotid artery 13 Left vagus nerve 14 Aortic arch 15 Esophagus 16 Esophageal plexus 17 Thoracic aorta 18 Left phrenic nerve 19 Pericardium at the central tendon of diaphragm 20 Right pulmonary artery 21 Left pulmonary artery 22 Tracheal lymph nodes 23 Superior tracheobronchial lymph nodes 24 Bronchopulmonary lymph nodes Bronchial tree in situ (ventral aspect). Heart and pericardium have been removed; the bronchi of the bronchopulmonary segments are dissected. 1–10 = numbers of segments (cf. p. 246 and 251). 15 12 22 6 11 5 2 1 14 2 23 1 3 21 3 20 24 4 5 4 17 8 5 6 6 15 8 7 8 9 9 10 10 Relation of aorta, pulmonary trunk, and esophagus to trachea and bronchial tree (schematic drawing). 1–10 = numbers of segments (cf. p. 246 and 251). 104750_S_265_290_Kap_4:_ 05.01.2010 10:43 Uhr Seite 276 276 Posterior Mediastinum: Mediastinal Organs Mediastinal organs (ventral aspect). The heart with the pericardium has been removed, and the lungs and aortic arch have been slightly reflected to show the vagus nerves and their branches. 1 Supraclavicular nerves 12 Right pulmonary artery 24 Left vagus nerve 2 Right internal jugular vein with ansa cervicalis 13 Right pulmonary veins 25 Left common carotid artery -
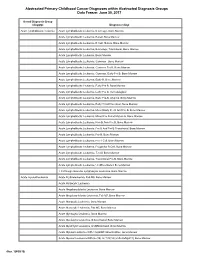
Clinical Content Data Points Dictionary
Abstracted Primary Childhood Cancer Diagnoses within Abstracted Diagnosis Groups Data Freeze: June 30, 2017 Broad Diagnosis Group (diaggrp) Diagnosis (diag) Acute lymphoblastic leukemia Acute Lymphoblastic Leukemia, B Lineage, Bone Marrow Acute Lymphoblastic Leukemia, B-Cell, Bone Marrow Acute Lymphoblastic Leukemia, B-Cell, Mature, Bone Marrow Acute Lymphoblastic Leukemia, B-Lineage, Transitional, Bone Marrow Acute Lymphoblastic Leukemia, Bone Marrow Acute Lymphoblastic Leukemia, Common , Bone Marrow Acute Lymphoblastic Leukemia, Common Pre B, Bone Marrow Acute Lymphoblastic Leukemia, Common, Early Pre B, Bone Marrow Acute Lymphoblastic Leukemia, Early B, Bone Marrow Acute Lymphoblastic Leukemia, Early Pre B, Bone Marrow Acute Lymphoblastic Leukemia, Early Pre B, Hematological Acute Lymphoblastic Leukemia, Early Pre-B, Atypical, Bone Marrow Acute Lymphoblastic Leukemia, Early T Cell Precursor, Bone Marrow Acute Lymphoblastic Leukemia, Mixed Early Pre B And Pre B, Bone Marrow Acute Lymphoblastic Leukemia, Mixed Pre B And Mature B, Bone Marrow Acute Lymphoblastic Leukemia, Non B, Non Pre B, Bone Marrow Acute Lymphoblastic Leukemia, Pre B And Pre B Transitional, Bone Marrow Acute Lymphoblastic Leukemia, Pre-B, Bone Marrow Acute Lymphoblastic Leukemia, Pre-T Cell, Bone Marrow Acute Lymphoblastic Leukemia, Progenitor B Cell, Bone Marrow Acute Lymphoblastic Leukemia, T-Cell, Bone Marrow Acute Lymphoblastic Leukemia, Transitional Pre B, Bone Marrow Acute Lymphoblastic Leukemia, Undifferentiated, Bone Marrow T Cell Large Granular Lymphocytic