The Impossibility of Informed Consent?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Whose Life Is It Anyway? a Study in Respect for Autonomy
Journal ofmedical ethics 1995; 21: 179-183 J Med Ethics: first published as 10.1136/jme.21.3.179 on 1 June 1995. Downloaded from Medicine and literature Whose Life Is It Anyway? A study in respect for autonomy Margaret Norden Marymount University, Arlington, Virginia, USA Abstract Precis of the story Brian Clark's drama, Whose Life Is It Anyway? (1), Whose Life Is It Anyway? opens with the explores the difficulties ofapplying the principle of hospitalization of Ken Harrison who was critically respect for autonomy to real-life circumstances. In the injured in an automobile accident four months play a permanently disabled patient, who wishes to be earlier and permanently paralyzed from the neck allowed to die, raises moral questions about the down. Although Ken arrived in critical condition, he adequacy of the autonomous agent, respect for the has since been stabilized, informed by his doctors autonomy ofothers, the authority of the law, the that his paralysis is permanent, and advised that he allocation ofsociety's resources, and the intrinsic value will be transferred to a long-term care facility. Facing ofhuman life. After a brief review of the story and the prospect of such an existence, Ken chooses not definition of respect for autonomy, this paper cites to live. He realizes that he could not survive outside passages from the play that dramatize the tension of an institution, and therefore, requests a discharge. between respect for autonomy and these other moral Although a psychiatrist confirms Ken's mental copyright. concerns. There follows a review of relevant competence, the hospital doctors refuse to discharge commentary from the classicists Kant and Mill and the him. -

American Psychiatric Association the Principles of Medical Ethics
American Psychiatric Association The Principles of Medical Ethics With Annotations Especially Applicable to Psychiatry 2013 Edition Copyright © 2010 American Psychiatric Association ALL RIGHTS RESERVED Manufactured in the United States of America 08 07 06 3 2 1 The Principles of Medical Ethics 2013 Edition (Previous editions 1973, 1978, 1981, 1984, 1985, 1989, 1992, 1993, 1995, 1995 Revised, 1998, 2001, 2001 Revised, 2006, 2008, 2009. 2009 Revised, and 2010 American Psychiatric Association 1000 Wilson Boulevard #1825 Arlington, VA 22209 THE PRINCIPLES OF MEDICAL ETHICS With Annotations Especially Applicable to Psychiatry 2013 Edition In 1973, the American Psychiatric Association (APA) published the first edition of The Principles of Medical Ethics with Annotations Especially Applicable to Psychiatry. Subsequently, revisions were published as the APA Board of Trustees and the APA Assembly approved additional annotations. In July of 1980, the American Medical Association (AMA) approved a new version of the Principles of Medical Ethics (the first revision since 1957), and the APA Ethics Committee1 incorporated many of its annotations into the new Principles, which resulted in the 1981 edition and subsequent revisions. This version includes changes to the Principles approved by the AMA in 2001. Foreword ALL PHYSICIANS should practice in accordance with the medical code of ethics set forth in the Principles of Medical Ethics of the American Medical Association. An up-to-date expression and elaboration of these statements is found in the Opinions and Reports of the Council on Ethical and Judicial Affairs of the American Medical Association.2 Psychiatrists are strongly advised to 3 be familiar with these documents. However, these general guidelines have sometimes been difficult to interpret for psychiatry, so further annotations to the basic principles are offered in this document. -

Clinician Conscience Objection and Ethical Care of Patients
1 VERMONT MEDICAL SOCIETY RESOLUTION 2 3 Clinician Conscience Objection and Ethical Care of Patients 4 5 As adopted at the VMS Annual Meeting on November 2, 2019 6 7 RESOLVED, that the VMS Policy on Physician Conscience be amended to read: The 8 Vermont Medical Society recognizes that the provision of certain types of medical care 9 or treatment can come in conflict with members’ cultural values, ethics or religious 10 beliefs. The Vermont Medical Society commits to protecting its members’ (physicians, 11 physician assistants, medical students) freedom to follow their own conscience in 12 deciding whether to participate in providing care or treatment, consistent with the 13 ethical norms of their professions, stated for physicians in AMA Code of Ethics Section 14 1.1.7, found in its entirety below, including fidelity to patients, respect for patient self- 15 determination, non-discrimination and informing the patient about all relevant options 16 for treatment, including options to which the physician morally objects, and information 17 regarding how to access such services. 18 19 AMA Code of Medical Ethics Opinion 1.1.7 20 Physicians are expected to uphold the ethical norms of their profession, including fidelity 21 to patients and respect for patient self-determination. Yet physicians are not defined 22 solely by their profession. They are moral agents in their own right and, like their 23 patients, are informed by and committed to diverse cultural, religious, and philosophical 24 traditions and beliefs. For some physicians, their professional calling is imbued with their 25 foundational beliefs as persons, and at times the expectation that physicians will put 26 patients’ needs and preferences first may be in tension with the need to sustain moral 27 integrity and continuity across both personal and professional life. -

The Hippocratic Oath and Principles of Medical Ethics
MEDICINE AND PUBLIC POLICY The Hippocratic Oath and Principles of Medical Ethics Gilbert Berdine MD The Hippocratic Oath is associated with the morally right. This tradition remains in the modern practice of medicine, but over time fewer medical era. “As God is my witness I hereby pledge to …” can graduates have taken any form of the Hippocratic be found in modern rituals to stress the seriousness Oath. As of 2006, the State University of New York of purpose. Courts in the U.S. require prospective Upstate Medical School in Syracuse was the only witnesses to pledge their truthfulness: “Do you sol- U.S. medical school that administered the classic emnly swear or affirm that you will tell the truth, the version of the Hippocratic Oath to its graduates. The whole truth, and nothing but the truth, so help you Hippocratic Oath has been revised to make it more God?” This pledge becomes a source of contention in acceptable to modern schools, but the medical pro- a multi-cultural society as some members believe in fession no longer has a common set of promises that other deities. guide it. This article will look at the classic version of the Hippocratic Oath to see why it has been aban- Anyone who takes his or her religion seriously doned. Modern medical students wish to graduate would not pledge to a pagan god as this would be a into an ancient order of physicians, so they long for form of idolatry. This pledge is probably the main rea- a solemn ceremony, but it is difficult to craft a solemn son that the Oath has been abandoned in the modern ceremony that remains agreeable to a diverse group era, but what can take its place as a symbol of seri- of students. -

Glossary of Medical Ethics and Hospice Care
Center for Bioethics and Health Policy Glossary of Medical Ethics and Hospice Care Before defining the terms commonly used in medical ethics and hospice care, it might be reasonable to ask: What is medical ethics and how does ethics differ from morals? Morals relates to the principles of right and wrong behavior, i.e., a specific act is right or it is wrong, end of question: to murder is wrong. Ethics, on the other hand, is a system of moral principles that apply values and judgments to accepted professional standards of conduct. Medical ethics evolve/devolve, i.e., what may have been considered ethical behavior in the 19th century may not be considered ethical in the 21st century and vice versa, e.g., eugenics was accepted as ethical behavior in the early 20th century. The Tuskegee study was accepted from its inception in the mid 1930s until it was abruptly discontinued in 1972. Advanced Health Care Directive (Living Will) - a document in which a person gives advanced directions about medical care, or designates who should make medical decisions for the person if they should lose their decision making capacity, or both. There are two types: treatment directive (Living Will), and proxy/surrogate directives (Healthcare Power of Attorney). Allow Natural Death (Allow to Die) – involves the withholding or withdrawing of life- sustaining treatment that is judged by the patient or proxy to be excessively burdensome and/or of little or no benefit. It involves a decision to no longer prevent the underlying disease process or pathology to run its course. Assent – a concept that applies to minors and refers to their right to have their own perspective represented in all decision making about their health care. -

Downloadable%20File/New Published Maps and Institutional Affiliations
Swazo et al. Philosophy, Ethics, and Humanities in Medicine (2020) 15:7 https://doi.org/10.1186/s13010-020-00091-6 RESEARCH Open Access A Duty to treat? A Right to refrain? Bangladeshi physicians in moral dilemma during COVID-19 Norman K. Swazo1*, Md. Munir Hossain Talukder2 and Mohammad Kamrul Ahsan2 Abstract Background: Normally, physicians understand they have a duty to treat patients, and they perform accordingly consistent with codes of medical practice, standards of care, and inner moral motivation. In the case of COVID-19 pandemic in a developing country such as Bangladesh, however, the fact is that some physicians decline either to report for duty or to treat patients presenting with COVID-19 symptoms. At issue ethically is whether such medical practitioners are to be automatically disciplined for dereliction of duty and gross negligence; or, on the contrary, such physicians may legitimately claim a professional right of autonomous judgment, on the basis of which professional right they may justifiably decline to treat patients. Methods: This ethical issue is examined with a view to providing some guidance and recommendations, insofar as the conditions of medical practice in an under-resourced country such as Bangladesh are vastly different from medical practice in an industrialized nation such as the USA. The concept of moral dilemma as discussed by philosopher Michael Shaw Perry and philosopher Immanuel Kant’s views on moral appeal to “emergency” are considered pertinent to sorting through the moral conundrum of medical care during pandemic. Results: Our analysis allows for conditional physician discretion in the decision to treat COVID-19 patients, i.e., in the absence of personal protective equipment (PPE) combined with claim of duty to family. -

WMA Medical Ethics Manual
3rd edition 2015 2 1 WORLD MEDICAL ASSOCIATION Medical Ethics Manual World Medical Association Medical Ethics Medical Ethics Manual – Principal Features of Manual 3rd edition 2015 Medical student holding a newborn © Roger Ball/CORBIS 2 1 © 2015 by The World Medical Association, Inc. TABLE OF CONTENTS All rights reserved. Up to 10 copies of this document may be made for your non-commercial personal use, provided that Acknowledgments ...................................................................................4 credit is given to the original source. You must have prior written Foreword ........................................................................................................5 permission for any other reproduction, storage in a retrieval system or transmission, in any form or by any means. Requests Introduction ..................................................................................................7 for permission should be directed to The World Medical · What is medical ethics? Association, B.P. 63, 01212 Ferney-Voltaire Cedex, France; · Why study medical ethics? email: [email protected], fax (+33) 450 40 59 37. · Medical ethics, medical professionalism, human rights and of Contents Table Medical Ethics Manual – law This Manual is a publication of the World Medical Association. · Conclusion It was written by John R. Williams, Director of Ethics, ...14 WMA (2003-2006) Chapter One – Principal Features of Medical Ethics Medical Ethics Manual – Principal Features of · Objectives Cover, design and concept by Tuuli Sauren, · What’s special about medicine? INSPIRIT International Communications, Belgium. · What’s special about medical ethics? Production and concept by · Who decides what is ethical? World Health Communication Associates, UK. · Does medical ethics change? Pictures by Van Parys Media/CORBIS · Does medical ethics differ from one country to another? · The role of the WMA Cataloguing-in-Publication Data · How does the WMA decide what is ethical? Williams, John R. -
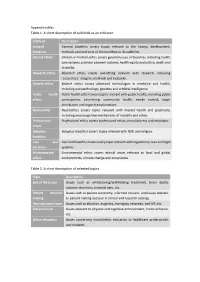
Table 1. a Short Description of Subfields As an Indicator. Table 2. A
Appendix tables Table 1. A short description of subfields as an indicator. Subfield Description General General bioethics covers topics relevant to the history, development, bioethics methods and overview of the bioethics or its subfields. Clinical ethics Clinical or medical ethics covers general issues of bioethics, including health care systems, provider payment systems, health equity and justice, death and disability. Research ethics Research ethics covers everything relevant with research, including researchers’ integrity, methods and biobanks. Biotech ethics Biotech ethics covers advanced technologies in medicine and health, including nanotechnology, genetics and artificial intelligence. Public health Public health ethics covers topics relevant with public health, including public ethics participation, advertising, community health, smoke control, organ distribution and organ transplantation. Neuroethics Neuroethics covers topics relevant with mental health and psychiatry, including neurocognitive mechanisms of morality and ethics. Professional Professional ethics covers professional ethics, moral distress and education. ethics Religious Religious bioethics covers topics relevant with faith and religion. bioethics Law and Law and bioethics covers every topic relevant with regulations, laws and legal bioethics systems. Environmental Environmental ethics covers ethical issues relevant to local and global ethics environments, climate change and ecosystems. Table 2. A short description of selected topics Topic Description End of life issues Issues such as withdrawing/withholding treatment, brain death, advance directives, terminal care, etc. Patient decision Issues such as patient autonomy, informed consent, and issues relevant making to patient making decision in clinical and research settings. Reproduction issues Issues such as abortion, eugenics, surrogacy, cesarean, and IVF, etc. Enhancement Issues relevant to physical and cognitive enhancement, moral enhance, etc. -

Informed Consent
AMA | JAMA Network | AMA Store Informed Consent Informed consent is more than simply getting a patient to sign a written consent form. It is a process of communication between a patient and physician that results in the patient's authorization or agreement to undergo a specific medical intervention. In the communications process, you, as the physician providing or performing the treatment and/or procedure (not a delegated representative), should disclose and discuss with your patient: The patient's diagnosis, if known; The nature and purpose of a proposed treatment or procedure; The risks and benefits of a proposed treatment or procedure; Alternatives (regardless of their cost or the extent to which the treatment options are covered by health insurance); The risks and benefits of the alternative treatment or procedure; and The risks and benefits of not receiving or undergoing a treatment or procedure. In turn, your patient should have an opportunity to ask questions to elicit a better understanding of the treatment or procedure, so that he or she can make an informed decision to proceed or to refuse a particular course of medical intervention. This communications process, or a variation thereof, is both an ethical obligation and a legal requirement spelled out in statutes and case law in all 50 states. Patient autonomy is the overarching ethical consideration that forms the core of informed consent. The AMA’s Code of Medical Ethics states that physicians must provide relevant information to their patients. The full disclosure of relevant information to patients is intended to protect each patient’s right to self-determination, bodily integrity, and to protect his or her voluntariness in the healthcare decisionmaking process. -

Conscience, Virtue, Integrity and Medical Ethics
J7ournal ofmedical ethics, 1984, 10, 171-172 J Med Ethics: first published as 10.1136/jme.10.4.171 on 1 December 1984. Downloaded from Editorial Conscience, virtue, integrity and medical ethics It is sometimes claimed that 'new fangled' in their textbook on medical ethics Beauchamp and philosophical medical ethics is unnecessary and indeed Childress say: 'In general, conscience is a mode of that it is positively disadvantageous, leading too often thought about one's acts and their rightness or 'to abstract and inconclusive intellectual argument - wrongness, goodness or badness' (5). neither conducive to postprandial reflection nor Thus it is immediately clear that claims for the necessarily relevant to the insistent demands on the adequacy of a good conscience for medical ethics must busy practitioner throughout his day' (1). Particularly make clear which ofthese two concepts ofconscience is in medical education, as Osler so quaintly put it, 'What intended. If the former, non-thinking, non-rational have bright eyes red blood quick breath and taut faculty of conscience is intended, the problem of muscles to do with philosophy?'(2). Rather, clinicians conflict of conscience, whether intrapersonal or so often claim, all that's needed for medical ethics is a interpersonal, is left unamenable to reason; Dr A's sound conscience, good character, and integrity. conscience tells him to transfuse the Jehovah's Witness First, and vitally, it is important to affirm that few if regardless of her own views, Dr B's conscience tells any moral philosophers, let alone those who are him not to transfuse such a patient. Where standscopyright. -

Principles — Respect, Justice, Nonmaleficence, Beneficence
Principles — Respect, Justice, Nonmaleficence, Beneficence The focus of this perspective is on the four PRINCIPLES supported by or compromised by the question or issue at hand. Philosophers Tom Beauchamp and Jim Childress identify four principles that form a commonly held set of pillars for moral life. Respect for Persons/Autonomy Acknowledge a person’s right to make choices, to hold views, and to take actions based on personal values and beliefs Justice Treat others equitably, distribute benefits/burdens fairly. Nonmaleficence (do no harm) Obligation not to inflict harm intentionally; In medical ethics, the physician’s guiding maxim is “First, do no harm.” Beneficence (do good) Provide benefits to persons and contribute to their welfare. Refers to an action done for the benefit of others. • Draws on principles or pillars that are a part of American life – familiar to most people, although not by their philosophical term • Compatible with both outcome-based and duty-based theories (respect for persons and justice are duty-based, while nonmaleficence and beneficence are outcome-based). • Provides useful and fairly specific action guidelines • Offers an approach that is appropriate for general bioethics and clinical ethics • Requires weighing and balancing – flexible, responsive to particular situations • Lacks a unifying moral theory that ties the principles together to provide guidelines • Principles can conflict and the theory provides no decision-making procedure to resolve these conflicts • Difficult to weigh and balance various principles • Autonomy in some cultures refers to individual autonomy, while in others refers to group/family/community autonomy Adapted with permission from Laura Bishop, Ph.D., Kennedy Institute of Ethics, Georgetown University 21. -

What Is the Scope of Autonomy in Medical Practice?
What is the Scope of Autonomy in Medical Practice? S. Falahati (University of Glasgow) Correspondence – S. Falahati: [email protected] ABSTRACT Autonomy, literally meaning self-rule, is an essential ethical principle, especially within the field of medicine. An individual with autonomy can make decisions on the basis of reflection and deliberation [1]. In general philosophy, autonomy is thought of as “the ability to be one's own person, to live life according to rationale and purpose that are taken as one's own and not the consequence of controlling or distorting outside forces” [2]. Individual autonomy is a notion that cultivates both self-ownership and self-governance and is based on ‘respect for persons‘, which states that all persons have the right “to make their own choices and develop their own life plans” [3]. The scope of autonomy reflects how far- reaching autonomy is, and what its boundaries are. This paper analyses the scope of autonomy in order to achieve greater clarity, and asks if this ethical principle should be superior to the three other principles (beneficence, non-maleficence and justice) rather than just “prima facie”.[4] Indeed, if there were to be a single overriding principle at all, should it definitely be autonomy rather than any of the other three? These questions will be dealt with and explored with the aid of medical ethical scenarios which help illustrate the points raised. We will identify what the limitations of autonomy are, and how far-reaching it is, all within the medical context. Key Words : autonomy; medicine; independence Introduction Respect for autonomy is widely accepted as imperative in medical ethics within the Western world.