Efficient Entry to Both Enantiomers of Α-Halohydrins Employing Two
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upregulation of Peroxisome Proliferator-Activated Receptor-Α And
Upregulation of peroxisome proliferator-activated receptor-α and the lipid metabolism pathway promotes carcinogenesis of ampullary cancer Chih-Yang Wang, Ying-Jui Chao, Yi-Ling Chen, Tzu-Wen Wang, Nam Nhut Phan, Hui-Ping Hsu, Yan-Shen Shan, Ming-Derg Lai 1 Supplementary Table 1. Demographics and clinical outcomes of five patients with ampullary cancer Time of Tumor Time to Age Differentia survival/ Sex Staging size Morphology Recurrence recurrence Condition (years) tion expired (cm) (months) (months) T2N0, 51 F 211 Polypoid Unknown No -- Survived 193 stage Ib T2N0, 2.41.5 58 F Mixed Good Yes 14 Expired 17 stage Ib 0.6 T3N0, 4.53.5 68 M Polypoid Good No -- Survived 162 stage IIA 1.2 T3N0, 66 M 110.8 Ulcerative Good Yes 64 Expired 227 stage IIA T3N0, 60 M 21.81 Mixed Moderate Yes 5.6 Expired 16.7 stage IIA 2 Supplementary Table 2. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of an ampullary cancer microarray using the Database for Annotation, Visualization and Integrated Discovery (DAVID). This table contains only pathways with p values that ranged 0.0001~0.05. KEGG Pathway p value Genes Pentose and 1.50E-04 UGT1A6, CRYL1, UGT1A8, AKR1B1, UGT2B11, UGT2A3, glucuronate UGT2B10, UGT2B7, XYLB interconversions Drug metabolism 1.63E-04 CYP3A4, XDH, UGT1A6, CYP3A5, CES2, CYP3A7, UGT1A8, NAT2, UGT2B11, DPYD, UGT2A3, UGT2B10, UGT2B7 Maturity-onset 2.43E-04 HNF1A, HNF4A, SLC2A2, PKLR, NEUROD1, HNF4G, diabetes of the PDX1, NR5A2, NKX2-2 young Starch and sucrose 6.03E-04 GBA3, UGT1A6, G6PC, UGT1A8, ENPP3, MGAM, SI, metabolism -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Supplementary Methods
Supplementary methods Human lung tissues and tissue microarray (TMA) All human tissues were obtained from the Lung Cancer Specialized Program of Research Excellence (SPORE) Tissue Bank at the M.D. Anderson Cancer Center (Houston, TX). A collection of 26 lung adenocarcinomas and 24 non-tumoral paired tissues were snap-frozen and preserved in liquid nitrogen for total RNA extraction. For each tissue sample, the percentage of malignant tissue was calculated and the cellular composition of specimens was determined by histological examination (I.I.W.) following Hematoxylin-Eosin (H&E) staining. All malignant samples retained contained more than 50% tumor cells. Specimens resected from NSCLC stages I-IV patients who had no prior chemotherapy or radiotherapy were used for TMA analysis by immunohistochemistry. Patients who had smoked at least 100 cigarettes in their lifetime were defined as smokers. Samples were fixed in formalin, embedded in paraffin, stained with H&E, and reviewed by an experienced pathologist (I.I.W.). The 413 tissue specimens collected from 283 patients included 62 normal bronchial epithelia, 61 bronchial hyperplasias (Hyp), 15 squamous metaplasias (SqM), 9 squamous dysplasias (Dys), 26 carcinomas in situ (CIS), as well as 98 squamous cell carcinomas (SCC) and 141 adenocarcinomas. Normal bronchial epithelia, hyperplasia, squamous metaplasia, dysplasia, CIS, and SCC were considered to represent different steps in the development of SCCs. All tumors and lesions were classified according to the World Health Organization (WHO) 2004 criteria. The TMAs were prepared with a manual tissue arrayer (Advanced Tissue Arrayer ATA100, Chemicon International, Temecula, CA) using 1-mm-diameter cores in triplicate for tumors and 1.5 to 2-mm cores for normal epithelial and premalignant lesions. -

Development of Potent and Selective Inhibitors of Aldo−Keto Reductase
Article pubs.acs.org/jmc Development of Potent and Selective Inhibitors of Aldo−Keto Reductase 1C3 (Type 5 17β-Hydroxysteroid Dehydrogenase) Based on N-Phenyl-Aminobenzoates and Their Structure−Activity Relationships † § ‡ § † † † ‡ Adegoke O. Adeniji, , Barry M. Twenter, , Michael C. Byrns, Yi Jin, Mo Chen, Jeffrey D. Winkler,*, † and Trevor M. Penning*, † Department of Pharmacology and Center of Excellence in Environmental Toxicology, Perelman School of Medicine, University of Pennsylvania, 130C John Morgan Building, 3620 Hamilton Walk, Philadelphia, Pennsylvania 19104-6084, United States ‡ Department of Chemistry, University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104, United States *S Supporting Information ABSTRACT: Aldo−keto reductase 1C3 (AKR1C3; type 5 17β-hydroxy- steroid dehydrogenase) is overexpressed in castration resistant prostate cancer (CRPC) and is implicated in the intratumoral biosynthesis of testosterone and 5α-dihydrotestosterone. Selective AKR1C3 inhibitors are required because compounds should not inhibit the highly related AKR1C1 and AKR1C2 isoforms which are involved in the inactivation of 5α-dihydrotestosterone. NSAIDs, N-phenylanthranilates in particular, are potent but nonselective AKR1C3 inhibitors. Using flufenamic acid, 2-{[3-(trifluoromethyl)phenyl]amino}- benzoic acid, as lead compound, five classes of structural analogues were synthesized and evaluated for AKR1C3 inhibitory potency and selectivity. Structure−activity relationship (SAR) studies revealed that a meta-carboxylic acid group relative to the amine conferred pronounced AKR1C3 selectivity without loss of potency, while electron withdrawing groups on the phenylamino B-ring were optimal for AKR1C3 inhibition. Lead compounds did not inhibit COX-1 or COX-2 but blocked the AKR1C3 mediated production of testosterone in LNCaP-AKR1C3 cells. These compounds offer promising leads toward new therapeutics for CRPC. -
Data Supplement
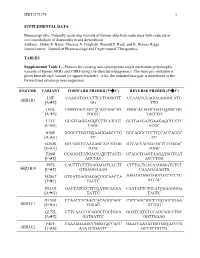
JPET#173179 1 SUPPLEMENTAL DATA Manuscript title: Naturally occurring variants of human aldo-keto reductases with reduced in vitro metabolism of daunorubicin and doxorubicin Authors: Onkar S. Bains, Thomas A. Grigliatti, Ronald E. Reid, and K. Wayne Riggs Journal name: Journal of Pharmacology and Experimental Therapeutics TABLES Supplemental Table 1—Primers for creating non-synonymous single nucleotide polymorphic variants of human AKRs and CBR4 using site-directed mutagenesis. The base pair mutation is given beneath each variant [in square brackets]. Also, the mutated base pair is underlined in the forward and reverse primer sequences. ENZYME VARIANT FORWARD PRIMER (5'3') REVERSE PRIMER (5'3') I15F CAAGATGCCCTTCCTGGGGTT CCAACCCCAGGAAGGGCATC AKR1B1 [AT] GG TTG H42L CGGGTACCGCCTCATCGACTG GGGCACAGTCGATGAGGCGG [AT] TGCCC TACCCG L73V GCGTGAGGAGGTCTTCATCGT GCTGACGATGAAGACCTCCTC [CG] CAGC ACGC K90E GGGCCTGGTGGAAGGAGCCTG GGCAGGCTCCTTCCACCAGGC [AG] CC CC G204S GCCAGTCCAAAAGCATCGTGG GTCACCACGATGCTTTTGGAC [GA] TGAC TGGC T288I CCAGGATATGACCATCTTACTC GTAGCTGAGTAAGATGGTCAT [CT] AGCTAC ATCCTGG P87S CACTTTCTTTGAGAGATCCCTT CTTTCCTCACAAGGGATCTCT AKR1B10 [CT] GTGAGGAAAG CAAAGAAAGTG M286T GTGATGAGGAGACGGCAACCA GAGTATGGTTGCCGTCTCCTC [TC] TACTC ATCAC N313D GACTATCCCTTCGATGCAGAA CAATATTCTGCATCGAAGGGA [AG] TATTG TAGTC R170H CCAACTTCAACCACAGGCAGC CTCCAGCTGCCTGTGGTTGAA AKR1C1 [GA] TGGAG GTTGG Q172L CTTCAACCGCAGGCTGCTGGA GGATCATCTCCAGCAGCCTGC [AT] GATGATCC GGTTGAAG F46Y CAATAGAAGCCGGGTACCACC GAATCAATATGGTGGTACCCG AKR1C2 [TA] ATATTTGATTC GCTTCTATTG JPET#173179 -

Metabolic Enzyme/Protease
Inhibitors, Agonists, Screening Libraries www.MedChemExpress.com Metabolic Enzyme/Protease Metabolic pathways are enzyme-mediated biochemical reactions that lead to biosynthesis (anabolism) or breakdown (catabolism) of natural product small molecules within a cell or tissue. In each pathway, enzymes catalyze the conversion of substrates into structurally similar products. Metabolic processes typically transform small molecules, but also include macromolecular processes such as DNA repair and replication, and protein synthesis and degradation. Metabolism maintains the living state of the cells and the organism. Proteases are used throughout an organism for various metabolic processes. Proteases control a great variety of physiological processes that are critical for life, including the immune response, cell cycle, cell death, wound healing, food digestion, and protein and organelle recycling. On the basis of the type of the key amino acid in the active site of the protease and the mechanism of peptide bond cleavage, proteases can be classified into six groups: cysteine, serine, threonine, glutamic acid, aspartate proteases, as well as matrix metalloproteases. Proteases can not only activate proteins such as cytokines, or inactivate them such as numerous repair proteins during apoptosis, but also expose cryptic sites, such as occurs with β-secretase during amyloid precursor protein processing, shed various transmembrane proteins such as occurs with metalloproteases and cysteine proteases, or convert receptor agonists into antagonists and vice versa such as chemokine conversions carried out by metalloproteases, dipeptidyl peptidase IV and some cathepsins. In addition to the catalytic domains, a great number of proteases contain numerous additional domains or modules that substantially increase the complexity of their functions. -

Biostatistics Mining Associated Method Identifies AKR1B10
www.nature.com/scientificreports OPEN Biostatistics mining associated method identifes AKR1B10 enhancing hepatocellular Received: 26 February 2018 Accepted: 25 June 2018 carcinoma cell growth and Published: xx xx xxxx degenerated by miR-383-5p Junqing Wang 1,2,3, Yunyun Zhou4, Xiaochun Fei5, Xuehua Chen2,3 & Yongjun Chen1 Previous studies have reported that the aberrantly expressed AKR1B10 is associated with many cancer development, however the functional roles of AKR1B10 and its regulatory mechanisms in hepatocellular carcinoma (HCC) have been limited studied. In this project, we identifed AKR1B10 functional as an oncogene in HCC through tumor/normal human tissue comparison from both GEO microarray and TCGA RNAseq dataset. Further experimental validations from three HCC cell lines (SMMC-7721, HePG2 and HeP3B) also suggested the ontogenetic functions of AKR1B10 in HCC tumor growth. By knocking down AKR1B10 through shRNA in HCC HeP3B cells, we showed it signifcantly induced cell cycle arrest and inhibited cell growth. Interestingly, integrative analysis of TCGA RNAseq data and miRNA-seq data predicted that miR-383-5p, a novel post-transcriptional tumor suppressor, is negatively associated with AKR1B10 expression. To further investigate the role of miR-383-5p in regulating AKR1B10 in HCC, we performed Dual-luciferase reporter assay experiments. Results showed that miR-383-5p is an upstream modulator targeting AKR1B10 in the post-transcriptional stage. Thus, we report AKR1B10 modulated regulated by miR-383-5p, promotes HCC tumor progress, and could be potentially a therapeutic target for precision medicine in HCC. World widely, hepatocellular carcinoma (HCC) presents a high incidence rate among human malignancies, rank- ing a ffh of morbidity and second of malignancy-related mortality1,2. -

Original Article Utilizing Network Pharmacology to Explore the Scientific Connotation of Processing Technology About Rehmanniae Radix Praeparata
Int J Clin Exp Med 2019;12(12):13504-13513 www.ijcem.com /ISSN:1940-5901/IJCEM0100588 Original Article Utilizing network pharmacology to explore the scientific connotation of processing technology about Rehmanniae Radix Praeparata Ruoqi Li, Guowei Zhou, Tianwei Xia, Yuxuan Wang, Chaoqun Ma, Jirong Shen Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 210029, Jiangsu, China Received August 5, 2019; Accepted October 8, 2019; Epub December 15, 2019; Published December 30, 2019 Abstract: Objective: Network pharmacology method was adopted in this study to establish differential component- target network of Rehmanniae Radix Praeparata before and after “steamed for nine times and dried for nine times”, explore the mechanism of the difference of pharmacological effect, reveal the scientific connotation of the process- ing method. Methods: The different components of Rehmanniae Radix Praeparata before and after “steamed for nine times and dried for nine times” were detected by consulting and screening the literature. PubChem database was used to convert all compounds to standard Canonical SMILES format. SMILES format files were imported into Swiss Target Prediction platform to predict potential targets for different components (possibility > 0). Targets with the highest confidence (score 0.900) were screened from STRING database to construct protein-protein interaction network. GO enrichment and KEGG pathway enrichment were analyzed by ClusterProfiler package in R language. Results: A total of 12 different components (catalpol, ajugol, Rehmannioside A, Acteoside, 5-hydroxymethy lfurfu- ral, Isoacteoside, stachyose, D(+)-Sucrose, raffinose, fructose, glucose and Manninotriose) were screened through literature review, and 101 potential targets were predicted. The GO analysis contained a total of 113 enrichment results. -

Table 3. PDB Representation of Gene Families A. H. Sapiens
Table 3. PDB representation of gene families A. H. -

Supplemental Figures 04 12 2017
Jung et al. 1 SUPPLEMENTAL FIGURES 2 3 Supplemental Figure 1. Clinical relevance of natural product methyltransferases (NPMTs) in brain disorders. (A) 4 Table summarizing characteristics of 11 NPMTs using data derived from the TCGA GBM and Rembrandt datasets for 5 relative expression levels and survival. In addition, published studies of the 11 NPMTs are summarized. (B) The 1 Jung et al. 6 expression levels of 10 NPMTs in glioblastoma versus non‐tumor brain are displayed in a heatmap, ranked by 7 significance and expression levels. *, p<0.05; **, p<0.01; ***, p<0.001. 8 2 Jung et al. 9 10 Supplemental Figure 2. Anatomical distribution of methyltransferase and metabolic signatures within 11 glioblastomas. The Ivy GAP dataset was downloaded and interrogated by histological structure for NNMT, NAMPT, 12 DNMT mRNA expression and selected gene expression signatures. The results are displayed on a heatmap. The 13 sample size of each histological region as indicated on the figure. 14 3 Jung et al. 15 16 Supplemental Figure 3. Altered expression of nicotinamide and nicotinate metabolism‐related enzymes in 17 glioblastoma. (A) Heatmap (fold change of expression) of whole 25 enzymes in the KEGG nicotinate and 18 nicotinamide metabolism gene set were analyzed in indicated glioblastoma expression datasets with Oncomine. 4 Jung et al. 19 Color bar intensity indicates percentile of fold change in glioblastoma relative to normal brain. (B) Nicotinamide and 20 nicotinate and methionine salvage pathways are displayed with the relative expression levels in glioblastoma 21 specimens in the TCGA GBM dataset indicated. 22 5 Jung et al. 23 24 Supplementary Figure 4. -

Functional and Structural Studies of AKR1B15 and AKR1B16: Two Novel Additions to Human and Mouse Aldo-Keto Reductase Superfamily
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Functional and structural studies of AKR1B15 and AKR1B16: Two novel additions to human and mouse aldo-keto reductase superfamily Mem`oriapresentada per JOAN GIMENEZ´ DEJOZ per optar al Grau de Doctor en Bioqu´ımicai Biologia Molecular Treball realitzat al departament de Bioqu´ımicai Biologia Molecular de la Universitat Aut`onomade Barcelona, sota la direcci´odels Doctors SERGIO PORTE´ ORDUNA, JAUME FARRES´ VICEN´ i XAVIER PARES´ CASASAMPERA Sergio Port´eOrduna Jaume Farr´esVic´en Xavier Par´esCasasampera Joan Gim´enezDejoz Bellaterra, 17 de juny de 2016 2 Agra¨ıments En primer lloc vull agrair als caps del grup, el Dr. Jaume Farr´esi el Dr. Xavier Par´esl'oportunitat de posar un peu a la ci`encia,iniciar-me en la carrera cient´ıfica,els seus bons consells i l’obtenci´o final d'aquesta tesi. Tamb´evoldria agrair especialment a tots els ADHs amb qui he compartit laboratori, sense vosaltres aix`ono hauria estat possible. Gr`aciesper aguantar tants dies a les fosques! Al Sergio-せんせい, per tot el que m'has ensenyat durant aquests anys, la bona guia, direcci´o, les inacabables correccions, per ensenyar-me a ser cr´ıticamb la meva pr`opiafeina i intentar sempre ser rigor´osi auto exigent tant amb els experiments com amb la redacci´oi preparaci´ode les figures (repassar cada figura mil cops buscant defectes abans de donar-la per bona ja s'ha convertit en costum) i tots i cada un dels inacabables \Podries..."que tant hem trobat a faltar en la `epoca final. -

AKR1B10 Promotes Breast Cancer Cell Migration and Invasion Via Activation of ERK Signaling
www.impactjournals.com/oncotarget/ Oncotarget, Advance Publications 2017 AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signaling Jia Li1,*, Yuanwei Guo1,2,*, Lili Duan1,*, Xinglin Hu1,*, Xi Zhang1,3, Jian Hu1,2, Li Huang1,2, Rongzhang He1, Zheng Hu1, Weihao Luo1, Tan Tan1,2, Renbin Huang1, Duanfang Liao1,4, Yuan-Shan Zhu5 and Di-Xian Luo1,2 1Translational Medicine Institute, National & Local Joint Engineering Laboratory for High-throughput Molecular Diagnosis Technology, Affiliated to The First People’s Hospital of Chenzhou, University of South China, Chenzhou 423000, P.R. China 2Center for Clinical Pathology, Affiliated to The First People’s Hospital of Chenzhou 423000, P.R. China 3Department of Neurology, Affiliated to The First People’s Hospital of Chenzhou 423000, P.R. China 4School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, P.R. China 5Department of Clinical Pharmacology, Xiangya Hospital and Institute of Clinical Pharmacology, Central South University and Hunan Key Laboratory of Pharmacogenetics, Changsha, Hunan 410078, P.R. China *These authors have contributed equally to this work Correspondence to: Di-Xian Luo, email: [email protected] Keywords: breast cancer, AKR1B10, ERK Received: November 04, 2016 Accepted: March 01, 2017 Published: March 28, 2017 Copyright: Li et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC- BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Background: Aldo-keto reductase family 1, member B10 (AKR1B10), is known to be significantly induced in the cells of various cancers such as breast cancer.